Neurotology and Inner Ear Biology
Our research group investigates inner ear balance functions and its associated diseases with a strong translational approach. On the basic science level, we explore the cellular and molecular basis of fluid and tissue homeostasis in the inner ear that creates and maintains the proper “milieu” for the sense of balance.
Research Focus
On the clinical science level, we aim to apply this knowledge to develop new diagnostic methods and explore new treatment strategies for diseases that affect the sense of balance, such as Meniere’s disease, chronic vestibular loss, vestibular schwannoma, and genetic diseases of the endolymphatic sac.
Show publicationsResearch Projects
The endolymphatic sac (ES) is an epithelial portion of the inner ear that harbors critical functions for maintaining the physiologic “milieu” in the inner ear, which enables the sensory hair cells in the hearing and balance organs to function properly. Despite this critical role, it remains largely unknown how the ES accomplishes its functions on the cellular and molecular level. Several projects of our group use various gene and protein expression assays, to investigate the molecular basis of ion transport functions, immune responses, and cell proliferative features of the ES in the healthy ES, as well as in disease conditions, such as Meniere’s disease, endolymphatic sac tumors, and developmental ES abnormalities (ELST, LVAS). The goal is to achieve a better understanding of the molecular pathomechanisms that affect hearing and balance in these diseases.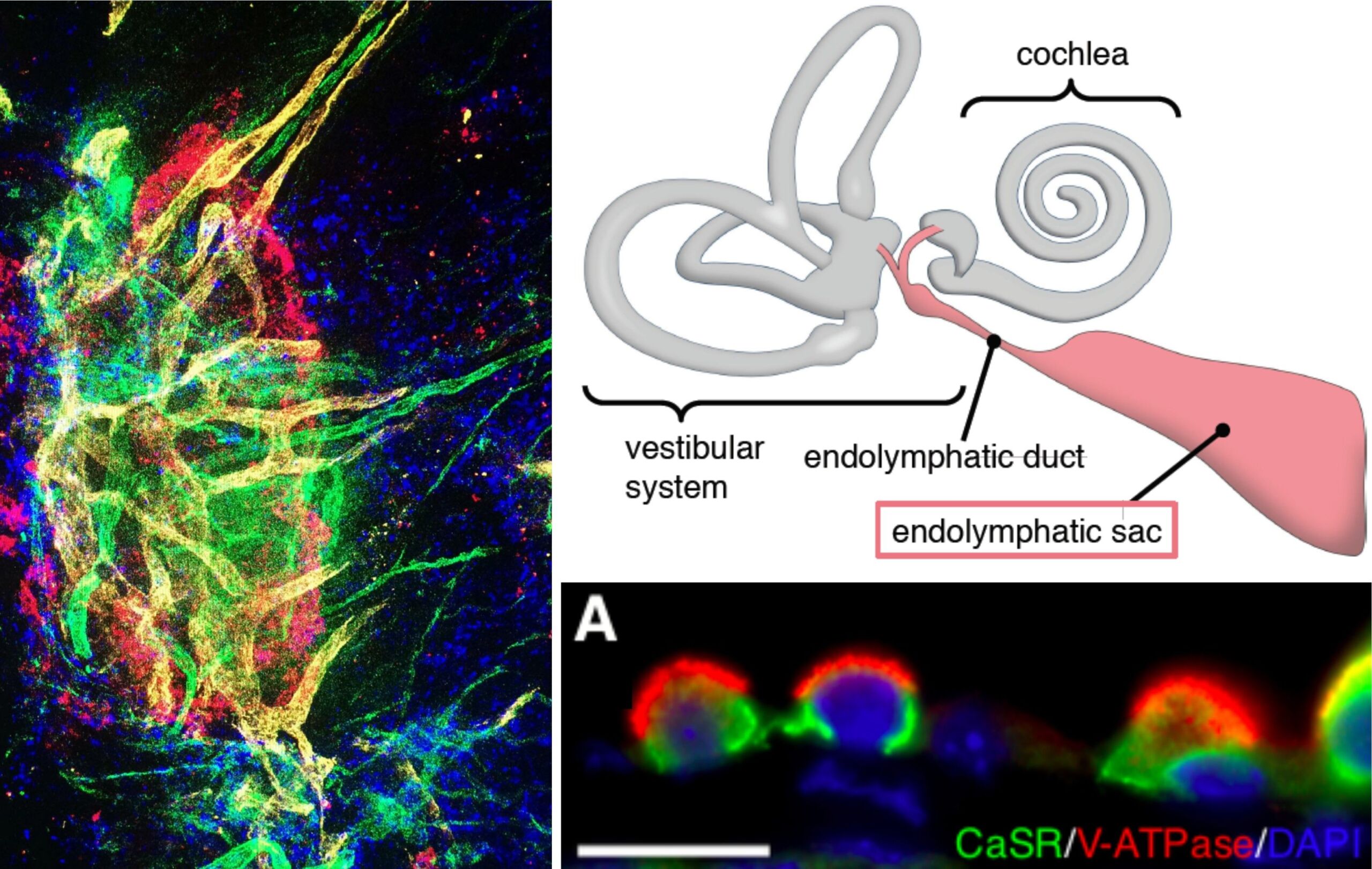
Meniere’s disease (MD) is a chronic degenerative inner ear disease. MD patients suffer from sudden episodes of vertigo, hearing loss, tinnitus and aural pressure. In the long term disease course, severe persistent hearing loss and chronic imbalance affect the patients’ quality of life. The underlying pathophysiology in the inner ear in MD remains unknown, and no causal treatment is available for patients. To facilitate research on this disease, we aim to establish the first animal model that fully recapitulates the pathological and clinical features of MD. In previous studies on primary diseased human (post mortem) inner ear tissue from MD patients, we identified a specific inner ear pathology that is presumably disease-causing. We surgically induce this pathology in adult mice, and investigate the development of MD symptoms (fluctuating vertigo, hearing loss, endolymphatic hydrops), using behavioral vestibular testing, brain stem audiometry, as well as histological and molecular biological (RNAseq) methods. The goal is to establish an animal model of MD that facilitates the search for new pharmacological treatments for MD.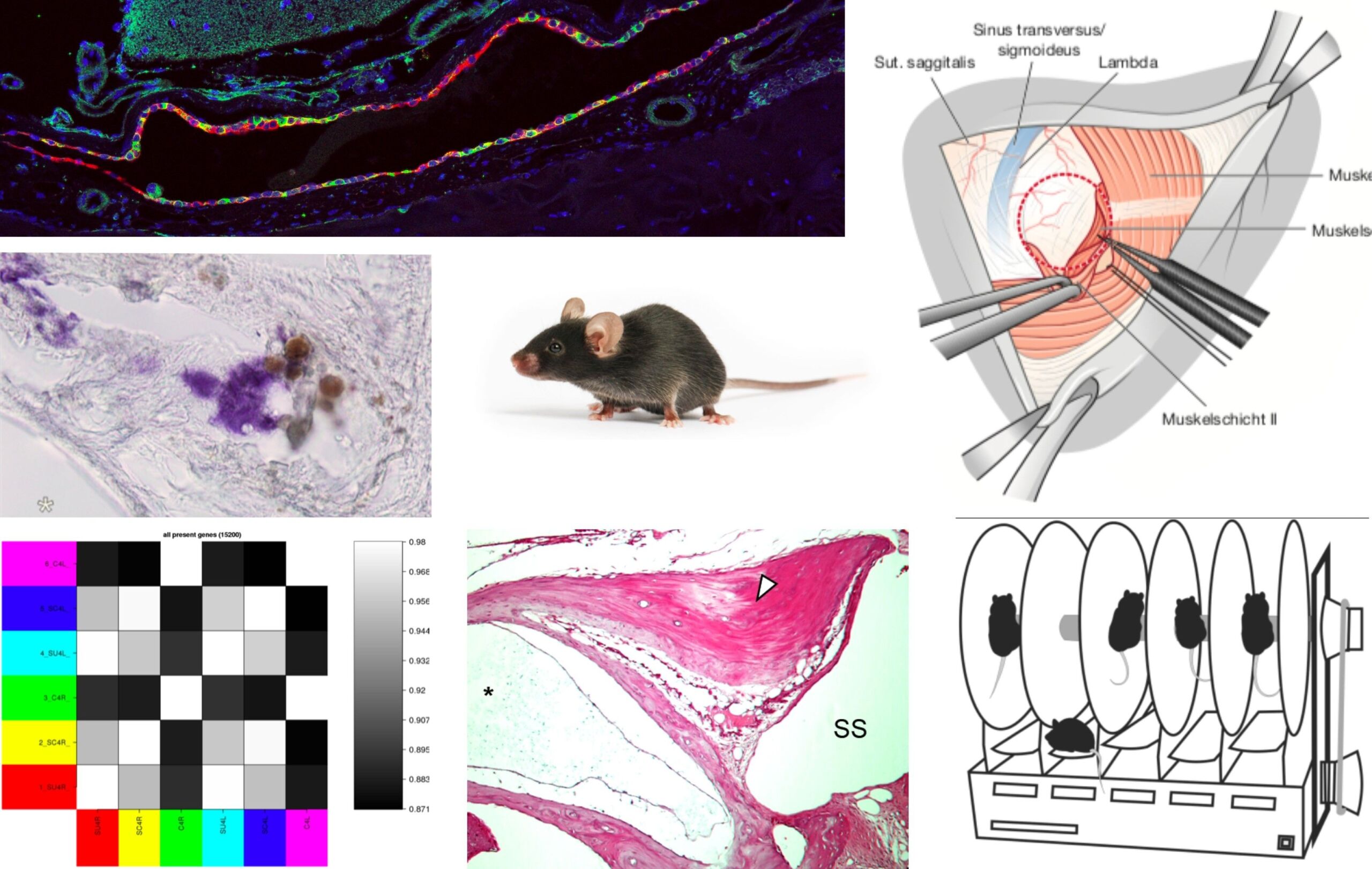
Patients with Meniere’s disease (MD) show heterogeneous clinical presentations with regard to their onset, frequency, quality, intensity, and time course of symptoms. This heterogeneity often delays the diagnostic process, and complicates the clinical management of individual patients, who respond differently to certain treatment regimens. In this project, we use gadolinium-enhanced MRI of the inner to distinguish two different endolymphatic sac (ES) pathologies (“endotypes”) in MD patients. Our preliminary data shows that these two endotype-specific patient groups demonstrate distinct clinical features. We therefore hypothesize that distinct “endotype-phenotype patterns” exist among MD patients, which may explain the observed clinical heterogeneity. In a ongoing prospective study, we further investigate the disease etiologies, the clinical presentations, and the disease course in patient groups, using clinical data analysis, morphometric analysis of Gd-MRI data, and genetic analysis (whole-exome sequencing). The goal is to elucidate the different etiologies of ES pathology and MD, and establish diagnostic markers that improve the processes of diagnosis-making and prognosticating the disease course in individual patients.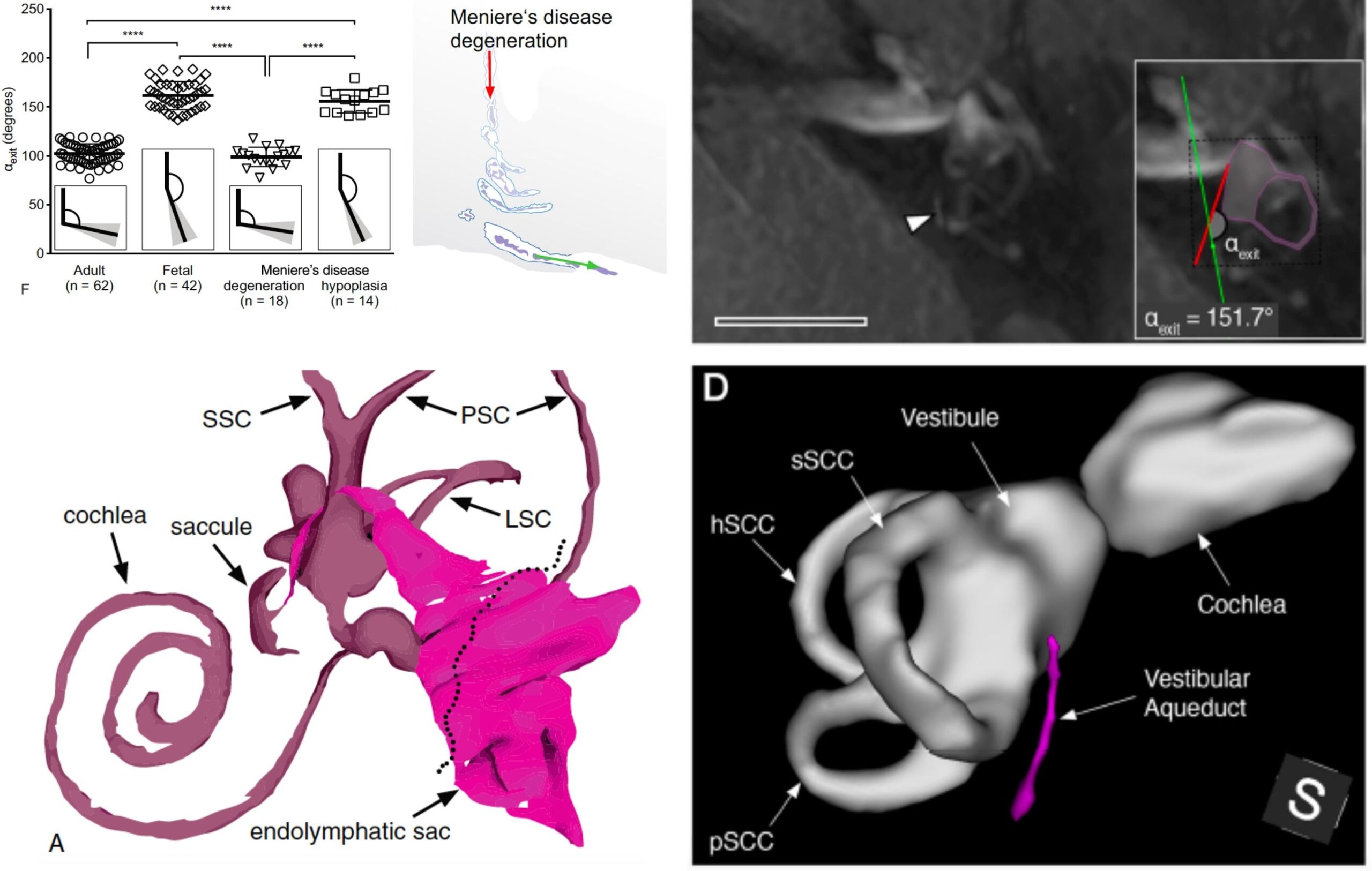
Bilateral persistent impairment or loss of vestibular function (chronic vestibulopathy, CV) can be caused by genetic factors, medication, or trauma. CV compromises the vestibulo-ocular reflex, which normally stabilizes our visual image during fast head movements, and thereby enables sharp vision, even while we are running, playing tennis, or riding a car on a bumpy road. Loss of the VOR in CV patients leads to jumping images (oscillopsia), and motion-induced blurry vision, which causes imbalance and increases the risk for falls. Our group, in collaboration with the Vestibulo-Oculomotor-Lab (USZ) and the Department of Informatics (ETH, Zürich), investigates the use of customized virtual reality (VR) eyewear for the treatment of oscillopsia symptoms and impaired dynamic visual acuity in CV patients. The goal is to explore new innovative treatments that improve vision and the quality of life in chronic vestibulopathy.Resources of the research group comprise a laboratory with bench working spaces for rodent surgery, histological and immunohistological work, which include a surgical microscopes, and different light microscopes. The laboratory is also equipped with an audiometry chamber for brain stem audiometry and otoacoustic emissions measurements on rodent animals, as well as a behavioral vestibular testing battery for rodents. The research group maintains continuous collaborative access to fluorescence and laser scanning microscopes at the Center for Microscopy and Image Analysis (ZMB) of the University of Zurich (UZH). Histological tissue processing is performed in cooperation with the Institute of Veterinary Pathology of the UZH has been established.Patient recruiting for prospective clinical studies takes place at the interdisciplinary center for vertigo and balance disorders at the University Hospital Zurich.
Interested in a Master’s thesis or doctoral thesis project?
Please contact Dr. med. Andreas Eckhard
Recent developments in the field of stem cell biology allow to generate in vitro inner ear sensory epithelia and otic neurons through directed differentiation of pluripotent stem cells (PSCs). The scope of this project is to optimize PSC-derived sensory epithelial patches differentiation and to develop a scalable, reproducible, and robust in vitro models to study sensory organ development ototoxicity and regeneration.
The goal of this project is to evaluate the use of patient specific iPSC-derived sensory cell types for in vitro testing and personalized medicine approaches. Specifically, to evaluate variability in differentiation outcomes, drug responses, and the feasibility to targeting underlying genetic mutations by gene correction/gene therapy.
- Institute for Veterinary Pathology, UZH (Prof. A. Kipar)
- Institute of Anatomy, UZH (Prof. J. Loffing)
- Institute of Veterinary Physiology, UZH (Prof. J. Vogel)
- Department of Endocrinology, USZ (Prof. Ch. Schmid-Baumann)
- Vestibulo-Oculomotor Lab Zürich, USZ (Prof. D. Straumann, PD Dr. K. Weber)
- Institute of Pathology and Molecular Pathology, USZ (Dr. med. Niels Rupp)
- Epidemiology, Biostatistics and Prevention Institute, UZH
- Functional Genomics Center Zurich, ETH/UZH
- Department of Informatics, ETH Zürich (Prof. Christian Holz, Prof. Ottmar Hilliges)
- Otopathology Laboratory, Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, USA (Prof. J. B. Nadol jr., Prof. S. D. Rauch)
- Otology & Neurotology Group at the Pfizer-Universidad de Granada-Junta de Andalucia Centre for Genomics and Oncology Research, Spain (Prof. J. A. Lopez-Escamez)
- Dr. Karl Koehler, Boston Children Hospital, Harvard Med School, USA
- Dr. Heiko Locher, Leiden University Medical Center, The Netherlands
- Dr. Hans Ruedi Widmer, University of Bern, Switzerland
- Dr. Giorgia Girotto, University of Trieste, Italy

