Here at the USZ, you can have the scientifically based Stockholm3 blood test, which significantly improves the early detection of aggressive prostate cancer, carried out. It has been practiced in Sweden and Norway since 2017 and is now also available in other European countries.
Numerous scientific studies with a total of 75,000 participants have demonstrated the following advantages of the Stockholm3 test:
The Stockholm3 test
- is detecting more cases of aggressive prostate cancer,
- reduces the number of unnecessary prostate biopsies (removal of tissue from the prostate),
- detects aggressive cancer even in men with a low PSA (prostate-specific antigen).
Who can take the Stockholm3 test?
The test is suitable for men between the ages of 45 and 75 who have not yet had prostate cancer and whose PSA value is above 1.5 ng/ml.
Stockholm3 at the USZ
If you are interested in a Stockholm3 blood analysis, register now for a consultation.
How much does Stockholm3 cost?
The costs of the Stockholm3 test, all associated consultations relating to the test at our clinic and the PSA test (if necessary) are generally covered by health insurance. The Stockholm3 test is invoiced by the laboratory in Switzerland; the cost of the Stockholm3 test is CHF 664.20.
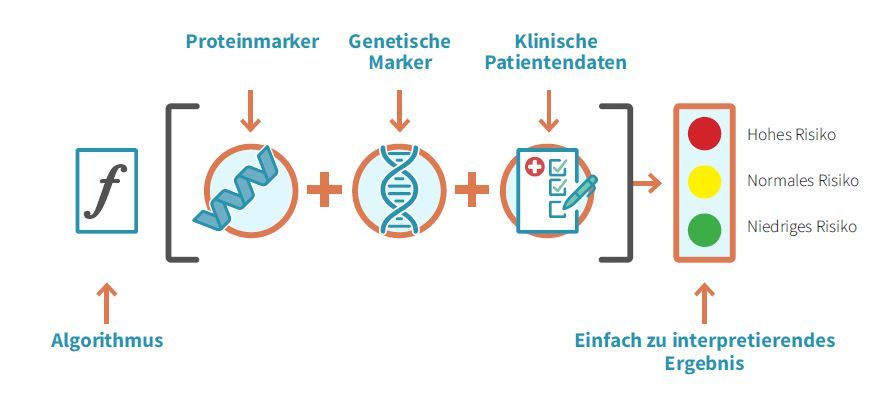
How does Stockholm3 work?
The Stockholm3 test is a blood test. This can be carried out from a PSA value of 1.5 ng/ml. Five different proteins and over 100 genetic biomarkers for the genetic risk profile are analyzed in the blood. The previous diagnostic standard, the PSA (prostate-specific antigen), is also determined. These laboratory values are combined with clinical data such as age, previous biopsies and family history in a risk score for the detection of aggressive prostate cancer at an early stage.
From a PSA value of 1.5 ng/ml, we can send blood samples from our patients to a Swiss laboratory. The treating urologist receives a clear treatment recommendation after the analysis. This may include, for example, that the risk of aggressive prostate cancer is low and that further screening in 1-6 years is sufficient. Or she recommends using further diagnostic procedures such as MRI (magnetic resonance imaging) or tissue removal from the prostate (biopsy) to clarify whether cancer could be present. All results are discussed with the patient and the next steps are decided.