Ventricular Arrhythmia Research – From Translational Models to Novel Therapeutic Strategies (Group leader: PD Dr. med. Ardan M. Saguner)
The research of the Saguner lab aims to improve the diagnosis and therapy of ventricular arrhythmias in rare electrical and structural heart diseases. Rapid advances in genetics and epigenetics of cardiovascular disease within the last years have led to a better understanding of the etiology of hereditary channelopathies and cardiomyopathies leading to ventricular arrhythmias and sudden cardiac death, and have improved deep phenotyping and personalized approaches for diagnosis, risk stratification and therapies of these complex entities.
Recent studies have stressed the importance of the combination of genetic, epigenetic and environmental factors to develop channelopathies and cardiomyopathies and to predict outcome. Therefore, our research focuses on the identification of novel pathogenic causative genetic variants and environmental factors that lead to disease manifestation, progression, arrhythmogenicity, variable disease penetrance and adverse outcome, as well as diagnostic tools such as disease-specific biomarkers (figure). Moreover, our work aims at improving the risk stratification process in these diseases. With regard to this, we work on the identification of clinical parameters that help to refine the prediction of potentially life-threatening ventricular arrhythmias and sudden cardiac death in these diseases, which often affect the young population. Lastly, we study novel therapeutic strategies such as stereotactic arrhythmia radioablation (STAR) and different exercise modalities in order to improve the disease course and reduce ventricular arrhythmia burden in structural heart disease.
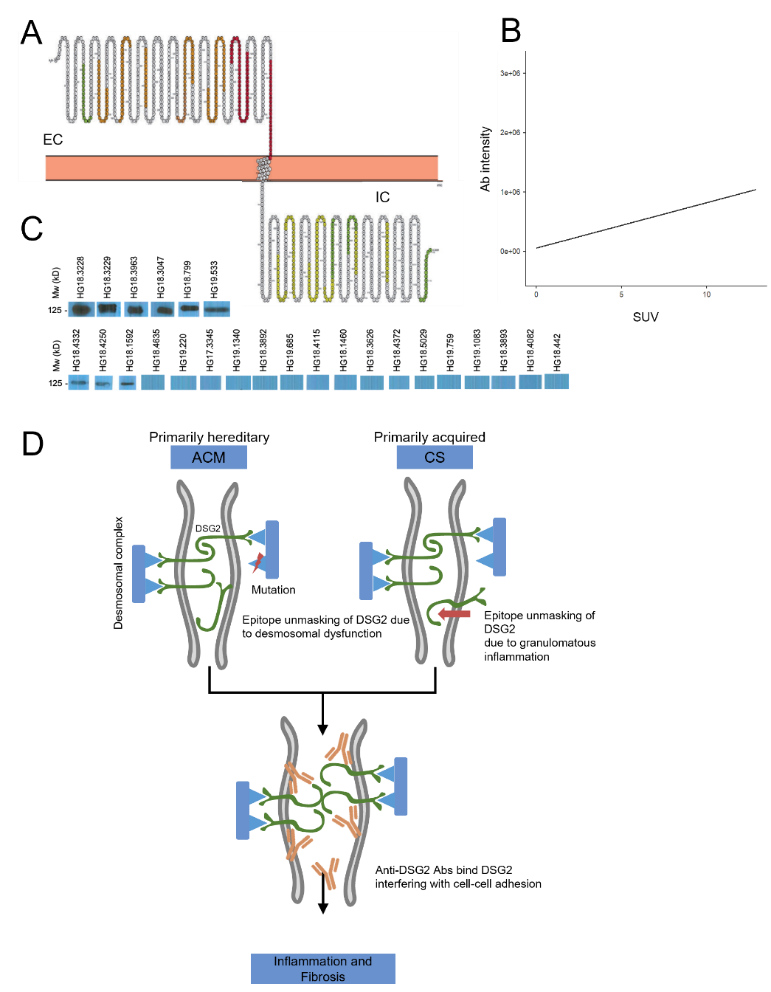
Figure. A). Serum binding intensity to desmoglein-2 oligopeptides is presented as colour grades onto a Protter (http://wlab.ethz.ch/protter) plot of the desmoglein-2 protein (red, strong binding; orange, moderate biding; yellow, weak binding; green, faint binding). Strongest binding is in regions of the fourth extracellular cadherin and extracellular anchor domains. EC=extracellular region, IC=cytoplasmic region. B). Pearson correlation coefficient (R) = 0.188 (p = 0.039) for PET inflammatory activity (SUV) vs. anti-DSG2 antibody (Ab) intensity assessed by pixel count of the western blot bands; C). Western blot bands of serum: anti-desmoglein-2 antibodies (anti-DSG2 Abs) were identified in 6 sarcoidosis patients (all showing cardiac involvement), whereas it was negative or below cut-off level (indicating unspecific binding) in the remaining 19/25 sarcoidosis patients; D). Central Illustration: Anti-DSG2 Abs in Arrhythmogenic Cardiomyopathy (ACM) and cardiac sarcoidosis (CS). In ACM (due to desmosomal instability) and in CS (due to granulomatous inflammation) the cadherin DSG2 epitopes are unmasked and exposed to the acquired immune system, inducing an autoimmune response to DSG2. This further promotes failure in cell crosslinking and enhances fibrosis, seen in both entities at later stages.
Ongoing research lines
- Assessing the consequences of PKP2 mutations on SCN5A function using high throughput automated planar patch clamp
- Identification of a proteomics signature to improve early diagnosis and risk stratification in arrhythmogenic cardiomyopathy
- Presence of anti-desmoglein2 autoantibodies in cardiac sarcoidosis and correlation with cardiac 18F-FDG positron-emission-tomography activity
- Characterization of arrhythmogenic activity during and after physical exercise in patients with arrhythmogenic cardiomyopathy
- Combined positron emission tomography – computed tomography immediately following ventricular tachyarrhythmia or electrical storm in patients with non-ischemic heart disease
- STOPSTORM: a prospective European validation cohort for stereotactic therapy of reentrant tachycardia
- Population-wide screens of the immune repertoire: a reverse personalized-medicine approach
Research Team
- Boldizsar Kovacs, MD
- Gonca Suna, MD PhD
- Valentina Rossi, MD
- Denny Lunk, MD
- Nicole R. Bonetti, MD
- Valon Gllareva
- Kai Thaler
Funding
- Schweizerische Herzstiftung/Swiss Heart Foundation
- Horizon 2020 European Commission
- Swiss Personalized Health Initiative
- Programmed ventricular stimulation as an additional primary prevention risk stratification tool in arrhythmogenic right ventricular cardiomyopathy: a multinational study. Gasperetti A, Carrick R, Costa S, Compagnucci P, Bosman L, Chivulescu M, Tichnell C, Murray B, Tandri H, Tadros R, Rivard L, van den Berg Maarten, Zeppenfeld K, Wilde A, Pompilio G, Carbucicchio A, dello Russo A, Casella M, Svensson A, Brunckhorst C, van Tintelen J, Platonov P, Haugaa K, Duru F, te Riele A, Khairy P, Tondo C, Calkins H, James C, Cadrin-Tourigny J, Saguner AM. Circulation. September 2022. online first.
- An autoantibody profile detects Brugada syndrome and identifies abnormally expressed myocardial proteins. Chatterjee D, Pieroni M, Fatah M, Charpentier F, Cunningham KS, Spears DA, Chatterjee D, Suna G, Bos JM, Ackerman MJ, Schulze-Bahr E, Dittmann S, Notarstefano PG, Bolognese L, Duru F, Hamilton RM, Saguner AM. Eur Heart J. 2020 Aug 7;41(30):2878-2890. doi: 10.1093/eurheartj/ehaa383.
- An autoantibody identifies arrhythmogenic right ventricular cardiomyopathy and participates in its pathogenesis. Chatterjee D, Fatah M, Akdis D, Spears DA, Koopmann TT, Mittal K, Rafiq MA, Cattanach BM, Zhao Q, Healey JS, Ackerman MJ, Bos JM, Sun Y, Maynes JT, Brunckhorst C, Medeiros-Domingo A, Duru F, Saguner AM, Hamilton RM. Eur Heart J. 2018 Nov 21;39(44):3932-3944. doi: 10.1093/eurheartj/ehy567.
- Impact of Genetic Variant Reassessment on the Diagnosis of Arrhythmogenic Right Ventricular Cardiomyopathy Based on the 2010 Task Force Criteria. Costa S, Medeiros-Domingo A, Gasperetti A, Akdis D, Berger W, James CA, Ruschitzka F, Brunckhorst CB, Duru F, Saguner AM. Circ Genom Precis Med. 2021 Feb;14(1):e003047. doi: 10.1161/CIRCGEN.120.003047.
- Benefits and outcomes of a new multidisciplinary approach for the management and financing of sudden unexplained death cases in a forensic setting in Switzerland. Neubauer J, Kissel CK, Bolliger SA, Barbon D, Thali MJ, Kloiber D, Bode PK, Kovacs B, Graf U, Maspoli A, Berger W, Haas C, Saguner AM. Forensic Sci Int. 2022 May;334:111240. doi: 10.1016/j.forsciint.2022.111240. Epub 2022 Feb 25.
- Differentiating hereditary arrhythmogenic right ventricular cardiomyopathy from cardiac sarcoidosis fulfilling 2010 ARVC Task Force Criteria. Gasperetti A, Rossi VA, Chiodini A, Casella M, Costa S, Akdis D, Büchel R, Deliniere A, Pruvot E, Gruner C, Carbucicchio C, Manka R, Dello Russo A, Tondo C, Brunckhorst C, Tanner F, Duru F, Saguner AM. Heart Rhythm. 2021 Feb;18(2):231-238. doi: 10.1016/j.hrthm.2020.09.015. Epub 2020 Sep 22.
- First magnetic resonance imaging-guided cardiac radioablation of sustained ventricular tachycardia. Mayinger M, Kovacs B, Tanadini-Lang S, Ehrbar S, Wilke L, Chamberlain M, Moreira A, Weitkamp N, Brunckhorst C, Duru F, Steffel J, Breitenstein A, Alkadhi H, Garcia Schueler HI, Manka R, Ruschitzka F, Guckenberger M, Andratschke N, Saguner AM. Radiother Oncol. 2020 Nov;152:203-207. doi: 10.1016/j.radonc.2020.01.008. Epub 2020 Feb 14.