Cardioimmunology (Group Leader: Prof. Dr. Burkhard Ludewig)
The immunological processes underlying myocardial disease are currently in the limelight of cardiology. In particular, immune checkpoint inhibitor-associated myocarditis and vaccine/virus-triggered inflammation of the myocardium pose challenges for diagnosis and treatment. The team of the Translational Cardioimmunology research group studies the interplay between immune and cardiac tissue cells with a particular focus on the role of fibroblasts in the myocardial inflammation.
Ongoing Research Lines
The gut-heart axis
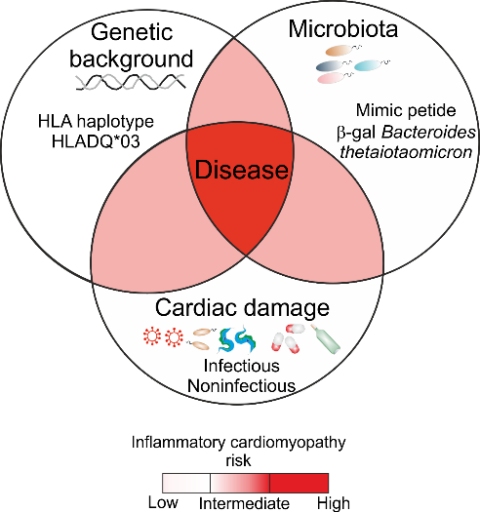
Autoimmune responses against the heart-specific protein myosin heavy chain 6 (MYH6) are a main driver for the progression of acute to chronic cardiac inflammation. Our group studies to which extent specific bacteria are linked to the development of myocarditis and the progression to chronic cardiac inflammation.
Improving early diagnosis of cardiac rejection after heart transplantation
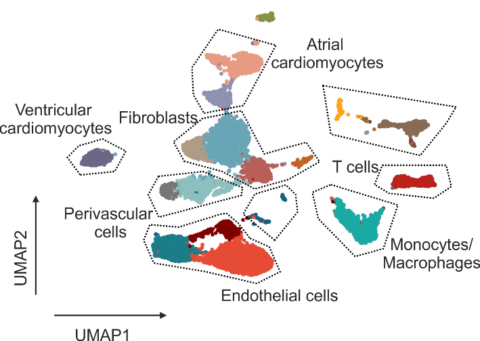
Rejection of donor hearts due to immune-mediated destruction of cardiac tissue integrity is a major challenge, both in terms of diagnosis and patient-adjusted immune-suppressive treatment. The current standard for the assessment of cardiac damage via histopathological examination of endomyocardial biopsies provides only limited information about the cellular composition and activation states in the inflamed tissue. Single cell transcriptomics-based deep phenotyping of transplant rejection represents a solution to this conundrum.
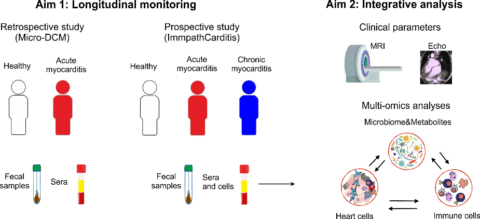
ImmpathCarditis
The majority of individuals with acute myocarditis recover completely; but approximately 30% of individuals with myocarditis do not recover and progress to potentially lethal inflammatory cardiomyopathy and heart failure. Preclinical studies of our group have revealed that the progression of myocarditis to lethal inflammatory cardiomyopathy is associated with the formation of autoimmune Th17 cells cross-reacting with bacteria in the intestinal microbiome and the expression of specific HLA haplotypes. To further investigate the association between specific HLA haplotypes and cardiac-reactive/microbiota-activated T cells during the progression of acute myocarditis, the “ImmpathCarditis” has been initiated.
Research Team
- Burkhard Ludewig, Prof. Dr.
- Dörthe Schmidt, PD PhD. Dr. med.
- Cristina del Carmen Gil-Cruz, PhD
- Kira Frischmann, MSc
- Anna Joachimbauer, Dr. med. univ.
- Matthias Nägele, Dr. med.
- Valentina Rossi, Dr. med.
Funding
- Baugarten Stiftung
- Blumenau-Léonie Hartmann-Stiftung
- ERC Advanced Grant 2021
- Hartmann Müller Stiftung
- Schweizerische Herzstiftung/Swiss Heart Foundation
- Cupovic J, Ring SS, Onder L, Colston JM, Lütge M, Cheng HW, De Martin A, Provine NM, Flatz L, Oxenius A, Scandella E, Krebs P, Engeler D, Klenerman P, Ludewig B. Adenovirus vector vaccination reprograms pulmonary fibroblastic niches to support protective inflating memory CD8+ T cells. Nat Immunol. 2021. https://doi.org/10.1038/s41590-021-00969-3
- Pikor NB, Mörbe U, Lütge M, Gil-Cruz C, Perez-Shibayama C, Novkovic M, Cheng HW, Nombela-Arrieta C, Nagasawa T, Linterman MA, Onder L, Ludewig B. Remodeling of light and dark zone follicular dendritic cells governs germinal center responses. Nat Immunol. 2020. https://doi.org/10.1038/s41590-020-0672-y
- Gil-Cruz C, Perez-Shibayama C, De Martin A, Ronchi F, van der Borght K, Niederer R, Onder L, Lütge M, Novkovic M, Nindl V, Ramos G, Arnoldini M, Slack EMC, Boivin-Jahns V, Jahns R, Wyss M, Mooser C, Lambrecht BN, Maeder MT, Rickli H, Flatz L, Eriksson U, Geuking MB, McCoy KD, Ludewig B. Microbiota-derived peptide mimics drive lethal inflammatory cardiomyopathy. 2019. https://doi.org/10.1126/science.aav3487
- Rieckmann M, Delgobo M, Gaal C, Büchner L, Steinau P, Reshef D, Gil-Cruz C, Horst ENT, Kircher M, Reiter T, Heinze KG, Niessen HW, Krijnen PA, van der Laan AM, Piek JJ, Koch C, Wester HJ, Lapa C, Bauer WR, Ludewig B, Friedman N, Frantz S, Hofmann U, Ramos GC. Myocardial infarction triggers cardioprotective antigen-specific T helper cell responses. J Clin Invest. 2019. https://doi.org/10.1172/JCI123859
- Nindl V, Maier R, Ratering D, De Giuli R, Züst R, Thiel V, Scandella E, Di Padova F, Kopf M, Rudin M, Rülicke T, Ludewig B. Cooperation of Th1 and Th17 cells determines transition from autoimmune myocarditis to dilated cardiomyopathy. Eur J Immunol. 2012. https://doi.org/10.1002/eji.201142209
- Onder L, Nindl V, Scandella E, Chai Q, Cheng HW, Caviezel-Firner S, Novkovic M, Bomze D, Maier R, Mair F, Ledermann B, Becher B, Waisman A, Ludewig B. Alternative NF-κB signaling regulates mTEC differentiation from podoplanin-expressing precursors in the cortico-medullary junction. Eur J Immunol. 2015. http://dx.doi.org/10.1002/eji.201545829