Work Packages
1: A phase I clinical trial with locally administered multifunctional mRNA-based CAR immune cells in glioblastoma
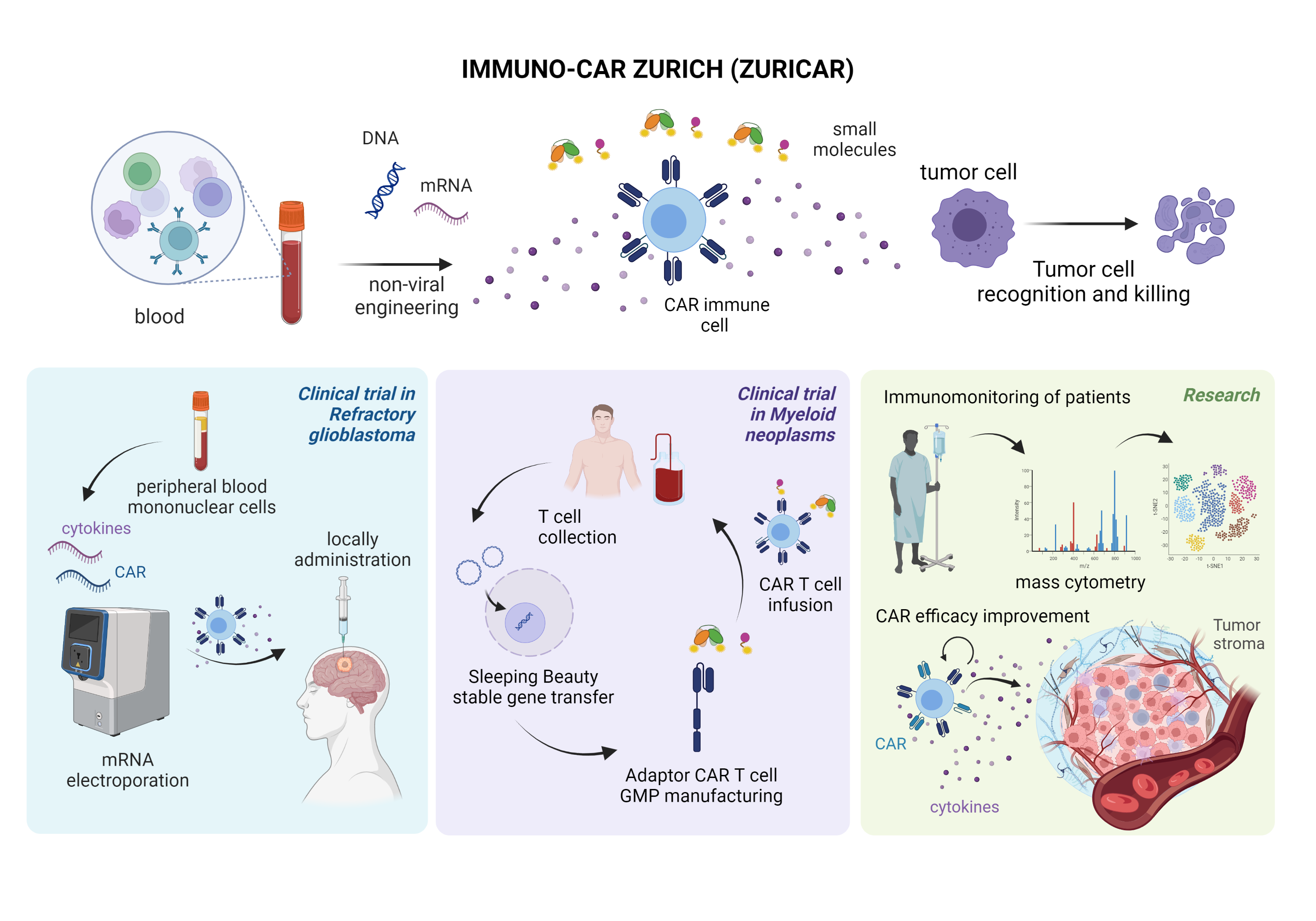
The first platform is based on highly efficient mRNA electroporation into non-activated, non-expanded peripheral blood mononuclear cells. This will enable a rapid clinical translation of innovative CAR products as it does not need cell activation or expansion and does not persistently change the genome of the cells. This platform will be applied within a phase I clinical trial to investigate the safety of local, intratumorally administered, multifunctional CAR PBMCs co-expressing a multi-targeting CAR and inflammatory cytokines in patients with refractory glioblastoma.
2: GMP production and clinical translation of Sleeping Beauty transposon engineered adaptor CAR T-cells
The second strategy is based on non-viral, switchable, multi-targeting adaptor CAR T-cells using the Sleeping Beauty transposon system in combination with small adaptor molecules. This platform will allow stable, cost-efficient transgene integration with increased safety based on on/off systems and increased efficacy by combinatorial targeting. CAR T cells will be manufactured in GMP by Wyss Zurich and will be evaluated pre-clinically before proceeding to a phase I/II trial. The first trial will be activated for patients with acute myeloid leukemia. Subsequently, we forseen application in solid tumors in combination with small molecules.
3: Research on CAR immunomonitoring and efficacy improvement
We will study the effect of CAR cell therapy using state-of-the-art technologies for immunomonitoring of patients including single-cell mass and flow cytometry of CAR immune cells and tumor ecosystems in samples from the clinical trials. Furthermore, we will pre-clinically explore strategies to boost Ad-CAR T cell efficacy by co-delivery of mRNA encoding for i) cytokines and ii) CAR molecules targeting the tumor stroma.
Overall, this project will address unmet needs for patients with difficult-to-treat malignancies and will spearhead innovative clinical CAR immune cell platforms at CCCZ.