Research Group Isabelle Arnold
Keywords
Eosinophils, gastrointestinal tract, inflammation, colorectal cancer, host defense
Summary & Mission statement
Our group studies how myeloid cells – and in particular eosinophils – control the balance between homeostasis and inflammation in the gastrointestinal tract. We aim to understand how “rewiring” the functional programs of these cells may improve the pathogenesis of inflammatory bowel diseases (IBD) and enhance anti-tumor responses in colorectal cancer.
Overview
The gastrointestinal (GI) tract harbors the largest number of immune cells in the body that are separated from our microbial residents by just a single layer of epithelial cells. The highly specialized mucosal immune system ensures the development of appropriate effector responses against pathogens while promoting tolerance toward innocuous commensal bacteria. Imbalance in this delicate equilibrium leads to a breakdown of intestinal homeostasis, resulting in chronic inflammation and associated malignancies.
A substantial population of eosinophils resides in the healthy GI lamina propria. We and others have described their pivotal contribution to the regulation of intestinal immune responses and protection against invading pathogens. Far from their traditional portray as terminal effector cells with limited plasticity, we find that eosinophil functions in the GI tract are highly context-dependent and are actively shaped by the local microenvironment. Our group thus investigates the pathways used by eosinophils to sense activating cytokine and bacterial signals, and the mechanisms through which these signals translate into a functional response in models of colitis and colorectal cancer. Our overall objective is to manipulate eosinophil activities to develop new therapeutic approaches.
Current research projects in our lab include:
1. Eosinophil plasticity and heterogeneity in colorectal cancer development
2. Promotion of cancer immunity
3. Eosinophil response to commensals and protection against pathogens
High intra-tumoral eosinophil infiltration correlates with better patient survival in colorectal cancer
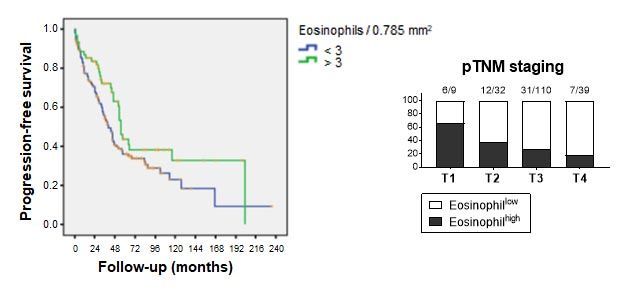
Eosinophil densities were quantified on a tissue microarray comprising 240 well annotated therapy-naïve colorectal cancer cases. A. Patients with high intra-tumoral eosinophil infiltration (>3 Eos/0.785mm2) had a significantly better progression-free survival than their eosinophillow counterparts (p=0.046, log rank Mantel-Cox test). B. High intra-tumoral eosinophil infiltration was inversely correlated with patient pathological tumor-node-metastasis (pTNM) status.