Research Group Didier Surdez
Keywords
Bone sarcoma, pediatric oncology, preclinical models, epigenetics, 3D chromatin organisation
Summary & Mission statement
Ewing Sarcoma, a pediatric bone tumor, is driven by a chimeric oncogene and few other recurrent mutations including STAG2, a cohesin member regulating the 3D chromatin organisation. Our mission is to decipher the functional consequences of these events to identify therapeutic vulnerabilities.
Overview
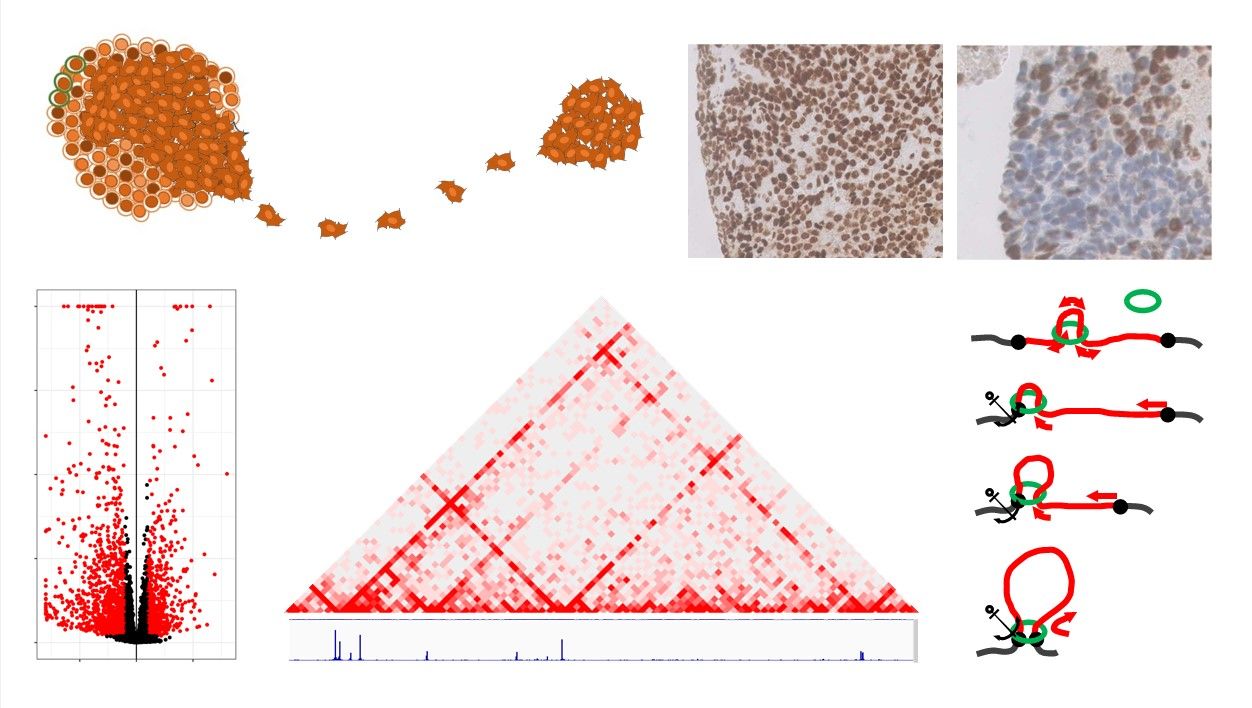
Ewing sarcoma is a bone tumor of childhood and adolescence/young adulthood which is characterized by a specific chromosomal translocation between EWSR1 and a gene of the ETS transcription factor family, most frequently FLI1. The resulting chimeric EWSR1-FLI1 oncogene acts as a pioneer transcription factor generating neo-enhancers which consequently massively rewires the transcriptome. We have identified that STAG2 LOF mutation is the most recurrent additional event in this cancer and is associated with a poor prognosis for these patients. STAG2 is a member of the ring-shaped cohesin complex which regulates, among other mechanisms, the 3D chromatin organisation. We recently showed that STAG2 LOF altered a dynamic process by which chromatin loops are generated and decreased promoter-(neo)enhancer interactions in these loops. This resulted in a broad transcriptional modulation and favored the migration and invasion of STAG2 LOF cells. Using faithful models, including de novo Ewing sarcoma models generated from mesenchymal stem cells, isogenic cellular models or patient derived models, we aim at identifying therapeutic vulnerabilities against these tumors using genetic and compound screening approaches. On a more fundamental perspective, we will further decipher how this mutation alters the dynamic organisation of the chromatin.
Publications