Research Group Beat W. Schäfer
Keywords
Sarcoma, oncogenic fusion transcription factors, precision medicine
Summary & Mission statement
Our mission is to improve diagnosis and treatment of pediatric sarcomas through improved understanding of the basic biology and functional precision medicine approaches.
Overview
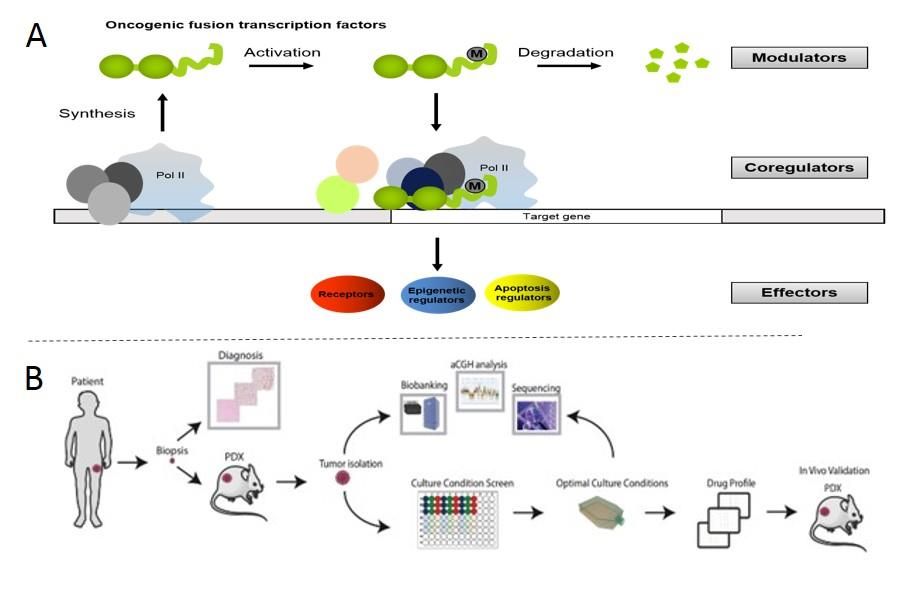
Prognosis of patients with pediatric sarcomas such as rhabdomyosarcoma and Ewing sarcoma are still unacceptably low, especially in case of relapse. In addition, high-dose chemotherapy as currently used can induce serious health problems later in life. Our laboratory uses two complementary approaches to overcome these limitations. First, we study the basic biology of pathognonomic oncogenic fusion transcription factors driving these diseases such as PAX3-FOXO1 and EWS-FLI1, with the long-term goal to identify possibilities for therapeutic blockade. We characterized several phosphorylation sites and their upstream kinases, whose inhibition by small molecules is now entering clinical trials. We have determined the interactome of the fusion proteins and characterized epigenetic mechanisms controlling accessibility to target gene enhancers. Currently, we implement several large-scale CRISPR/Cas9 screens to further deepen our understanding of the tumorigenic process. Second, we believe that for pediatric tumors with their generally low mutational burden sequencing efforts as currently implemented to identify actionable mutations have to be complemented by functional approaches. Therefore, our lab is establishing techniques for culturing and characterization of patient derived tumor cells with the aim to find tumor specific vulnerabilities independent of genomics. We generated a bank of patient-derived xenografts which are used to establish a drug-screening platform of both 2D and 3D cultures. These phenotypic screens coupled to in-depth molecular characterization are expected to provide critical predictive and mechanistical information to advance the field to the “postgenome” area.
Publications