Research Group: Cardiac regeneration (Group leader: Dr. med. Bernhard Haubner)
For a long time, the heart was considered a postmitotic organ with a lack of regenerative capacity, i.e., dead myocardium cannot be replaced with functional myocardial tissue. At first clinical glance, this fact has not changed. For some years now, there has been increasing scientific evidence that the heart does have a regenerative capacity. However, this is far from sufficient to compensate for clinically significant cardiac disease.
Together with other research groups, we succeeded in demonstrating an excellent regenerative capacity of newborn mouse hearts and demonstrated complete regenerative capacity after clinically relevant myocardial infarction. Additional evidence from other mammalian experimental models and even case reports of human babies confirm the great plasticity of neonatal mammalian hearts.
In our lab we address the questions of what are the molecular mechanisms of neonatal cardiac regeneration, and can we translate the answers to heal adult human hearts?
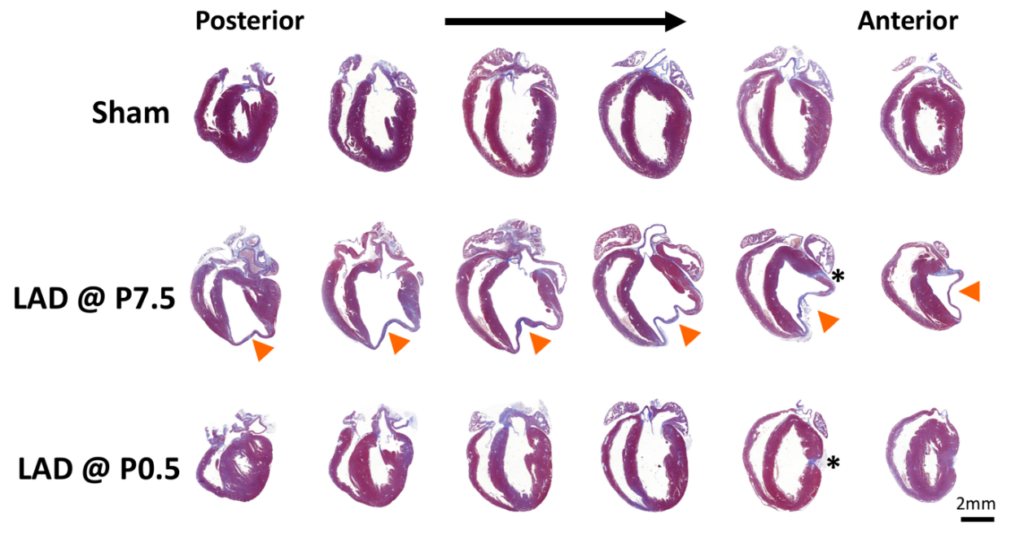
Figure. Serial sections cut longitudinally from the back- to the front wall of the hearts harvested 30 days post injury or sham surgery. Tissue sections were stained with trichrome and aniline blue; blue demarks fibrosis, whereas viable cardiomyocytes are brick red. Arrowheads point at the site of myocardial infarction in hearts ligated at postnatal day 7. Asterisks indicate the locations of the ligatures. LAD left anterior descending artery, P0.5 postnatal day 1, P7.5 postnatal day 7. Haubner BJ, Schuetz T, Penninger JM. Basic Res Cardiol. 2016.
Research Team
- Thomas Schütz, MD
- Theresa Dolejsi, MD
Funding
- ERA CVD network grant, FWF I 4161-B
- Costa A, Cushman S, Haubner BJ, Derda AA, Thum T, Bär C. Neonatal injury models: integral tools to decipher the molecular basis of cardiac regeneration. Basic Res Cardiol. 2022 May 3;117(1):26.
- Dolejsi T, Delgobo M, Schuetz T, Tortola L, Heinze KG, Hofmann U, Frantz S, Bauer A, Ruschitzka F, Penninger JM, Campos Ramos G, Haubner BJ. Adult T-cells impair neonatal cardiac regeneration. Eur Heart J. 2022 Apr 13:ehac188.
- Adamowicz M, Morgan CC, Haubner BJ, Noseda M, Collins MJ, Paiva MA, Srivastava PK, Gellert P, Razzaghi B, Game L, Bottolo L, Schneider MD, Harding SE, Penninger JM, Aitman TJ. Functionally conserved non-coding regulators of cardiomyocyte proliferation and regeneration in mouse and human. Circ Genom Precis Med. 2018 Feb.
- Haubner BJ, Schuetz T, Penninger JM. A reproducible protocol for neonatal ischemic injury and cardiac regeneration in neonatal mice. Basic Res Cardiol. 2016 Nov; 111(6):64.
- Polizzotti BD, Ganapathy B, Haubner BJ, Penninger JM, Kühn B. A cryoinjury model in neonatal mice for cardiac translational and regeneration research. Nat Protoc. 2016 Mar;11(3):542-52.
- Haubner BJ, Schneider J, Schweigmann U, Dichtl W, Velik-Salchner C, Stein JI, Penninger JM. Regeneration of human neonatal hearts after myocardial infarction. Circ Res. 2016 Jan 22;118(2):216-21.
- Polizzotti BD, Ganapathy B, Walsh S, Choudhury S, Ammanamanchi N, Bennet D, dos Remedios C, Haubner BJ, Penninger JM, Kuhn B. Stimulation of cardiomyocyte regeneration in neonatal mice and in human myocardium with neuregulin reveals a therapeutic window. Sci Transl Med. 2015 Apr 1;7(281):281ra45.
- Haubner BJ, Adamowicz-Brice M, Khadayate S, Tiefenthaler V, Metzler B, Aitman T, Penninger JM. Complete cardiac regeneration in a mouse model of myocardial regeneration. Aging (Albany NY). 2012 Dec;4(12):966-77.