Cardiac immunofibrosis
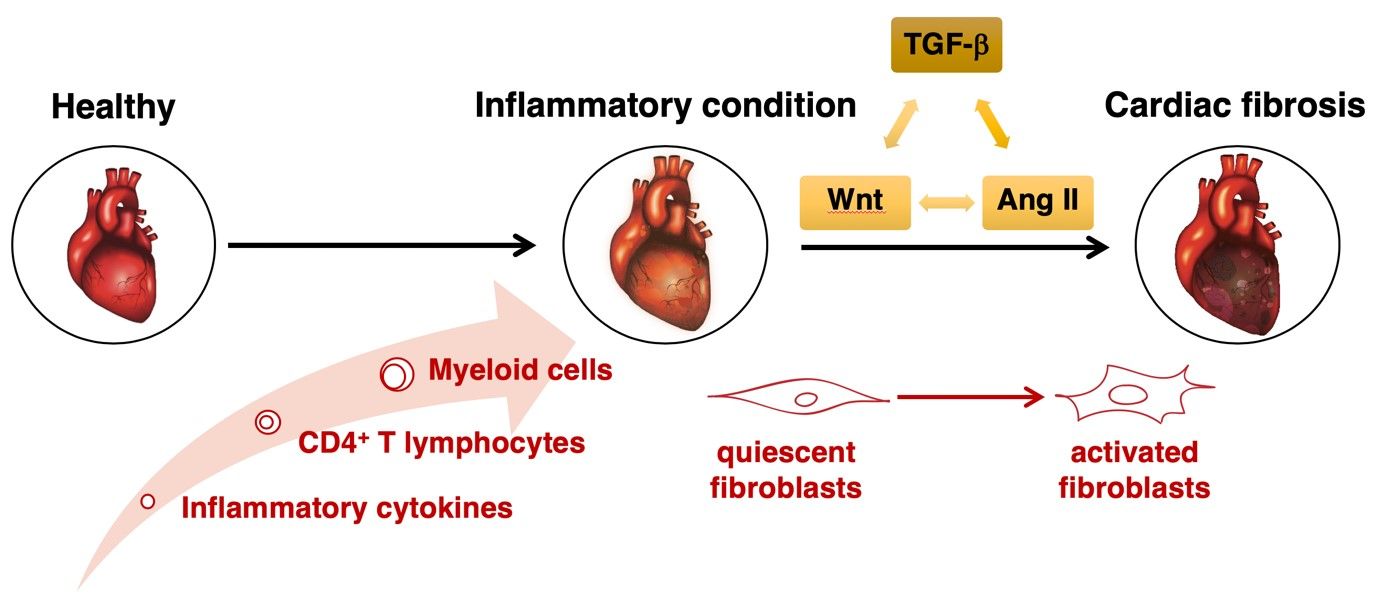
Cardiac fibrosis is a hallmark of pathogenic condition in the heart and promotes its dysfunction. In the process of fibrogenesis, physiological structure of the myocardium is replaced by an exaggerated accumulation of stromal cells and extracellular matrix components in the tissue. It has been recognized that local and systemic inflammation represent important pathogenic triggers of fibrotic processes in the heart.
Our research is focused on understanding how heart-specific and heart non-specific autoimmune responses contribute to these pathogenic processes. On cellular level, we investigate 1) a cross-talk between CD4+ T lymphocytes, myeloid cells and microvascular endothelial cells in induction of cardiac inflammation and 2) impact of inflammatory cells and inflammatory mediators on pathogenic activation of cardiac fibroblasts. On molecular level, we study 1) interplay between TGF-beta, Wnt and angiotensin II in the process of cardiac fibrogenesis and 2) cardioprotective aspects of TNF-alpha signaling. Furthermore, we are interested in the role of exosomes in intercellular communication. In our research we are using 1) in vivo mouse models of experimental autoimmune myocarditis and rheumatoid arthritis and 2) in vitro models of various primary mouse and human cardiac cells and 3D model of human cardiac microtissues.
By using these preclinical approaches, we aim to elucidate critical immune-mediated mechanisms controlling development of cardiac fibrosis and heart failure. We believe that outcomes of our research will contribute to better understanding the nature of immunofibrotic heart diseases and to development of novel treatment strategies.
- Kania G, Siegert S, Behnke S, Prados-Rosales R, Casadevall A, Luscher TF, Luther SA, Kopf M, Eriksson U, Blyszczuk P. Innate signaling promotes formation of regulatory nitric oxide-producing dendritic cells limiting T-cell expansion in experimental autoimmune myocarditis. Circulation. 2013;127:2285-94.
- Blyszczuk P, Muller-Edenborn B, Valenta T, Osto E, Stellato M, Behnke S, Glatz K, Basler K, Luscher TF, Distler O, Eriksson U, Kania G. Transforming growth factor-beta-dependent Wnt secretion controls myofibroblast formation and myocardial fibrosis progression in experimental autoimmune myocarditis. Eur Heart J. 2017;38:1413-25.
- Zarak-Crnkovic M, Kania G, Jazwa-Kusior A, Czepiel M, Wijnen WJ, Czyz J, Muller-Edenborn B, Vdovenko D, Lindner D, Gil-Cruz C, Bachmann M, Westermann D, Ludewig B, Distler O, Luscher TF, Klingel K, Eriksson U, Blyszczuk P. Heart non-specific effector CD4(+) T cells protect from postinflammatory fibrosis and cardiac dysfunction in experimental autoimmune myocarditis. Basic Res Cardiol. 2019;115:6.
- Tkacz K, Rolski F, Czepiel M, Dzialo E, Siedlar M, Eriksson U, Kania G, Blyszczuk P. Haploinsufficient Rock1(+/-) and Rock2(+/-) Mice Are Not Protected from Cardiac Inflammation and Postinflammatory Fibrosis in Experimental Autoimmune Myocarditis. Cells. 2020;9.
- Blyszczuk P, Zuppinger C, Costa A, Nurzynska D, Di Meglio FD, Stellato M, Agarkova I, Smith GL, Distler O, Kania G. Activated Cardiac Fibroblasts Control Contraction of Human Fibrotic Cardiac Microtissues by a beta-Adrenoreceptor-Dependent Mechanism. Cells. 2020;9.
- Dzialo E, Rudnik M, Koning RI, Czepiel M, Tkacz K, Baj-Krzyworzeka M, Distler O, Siedlar M, Kania G, Blyszczuk P. WNT3a and WNT5a Transported by Exosomes Activate WNT Signaling Pathways in Human Cardiac Fibroblasts. Int J Mol Sci. 2019;20.
- Stellato M, Czepiel M, Distler O, Blyszczuk P, Kania G. Identification and Isolation of Cardiac Fibroblasts From the Adult Mouse Heart Using Two-Color Flow Cytometry. Frontiers in Cardiovascular Medicine. 2019;6.
- Blyszczuk P, Szekanecz Z. Pathogenesis of ischaemic and non-ischaemic heart diseases in rheumatoid arthritis. RMD open. 2020;6.
- Blyszczuk P. Myocarditis in Humans and in Experimental Animal Models. Front Cardiovasc Med. 2019;6:64.
- Dzialo E, Tkacz K, Blyszczuk P. Crosstalk between the TGF-beta and WNT signalling pathways during cardiac fibrogenesis. Acta Biochim Pol. 2018;65:341-9.
