Medical physics of radiotherapy
Over the past 20 years, research and development in medical physics has improved the accuracy and conformity of radiotherapy tremendously. This includes the development of intensity-modulated radiotherapy (IMRT), which allows the delivery of highly conformal dose distributions to complex shaped tumors.
More recently, the development of image guided adaptive radiotherapy has provided means to correct for geometric changes and organ motion over the course of therapy. For a general introduction to the technology of modern precision radiotherapy, you may watch this presentation given at the Scientifica 2019 (in german). The medical physics group contributes to these technological advances of radiotherapy through both clinically applied and fundamental research projects.
We focus on 3 areas of research:
- Radiotherapy treatment planning: We conduct research on mathematical optimization methods for radiotherapy planning to further improve treatment planning systems. This includes both X-ray therapy as well as proton therapy.
- Target delineation and outcome prediction: Here, we focus on quantitative analysis of medical images such as MRI, CT and PET, with the goal of precisely defining the region to be irradiated and predicting the patient’s response to treatment. Work in this domain is integrated into the UZH Clinical Research Priority Program Artificial intelligence in oncological imaging.
- Adaptive radiotherapy: We further develop state-of-the-art systems to correct for motion of the tumor. Our department is the first in Switzerland to install a MRI-Linac, a combination of MRI scanner and radiotherapy device.
Projects
Group leader: Stephanie Tanadini-Lang
Group members: Marta Bogowicz, Hubert Gabryś, Diem Vuong
Cancer is a heterogeneous disease with respect to etiology, pathogenesis, therapy response and prognosis. Tumor response to therapy varies not only among patients but also within the tumor itself. Today, increasing number of cancer treatment options are available due to rapid technical developments. Therefore, decision support systems are needed to offer the right treatment to the right patient.
One possibility to optimize treatment strategies is the identification of biomarkers. In recent years, imaging has become increasingly important due to its non-invasive nature for the identification of new prognostic biomarkers. Imaging datasets are expected to hold more information than visible to the human eye. Radiomics describes the extraction of a large number of meaningful quantitative features from medical images, such as computed tomography, positron emission tomography or magnetic resonance imaging. Using our in-house developed radiomics software (Z-Rad) we can extract more than 1’000 radiomic features describing tumor shape, tumor intensity, tumor texture from medical images (figure). Based on mathematical definitions we investigate tumor morphology as well as the prominent perceptual texture characteristics such as regularity (or periodicity), directionality and complexity. These radiomic features are potential biomarkers of the cancer phenotype, and hence can be used for patient outcome prognosis or for correlation to the tumor biology using advanced statistical methods.
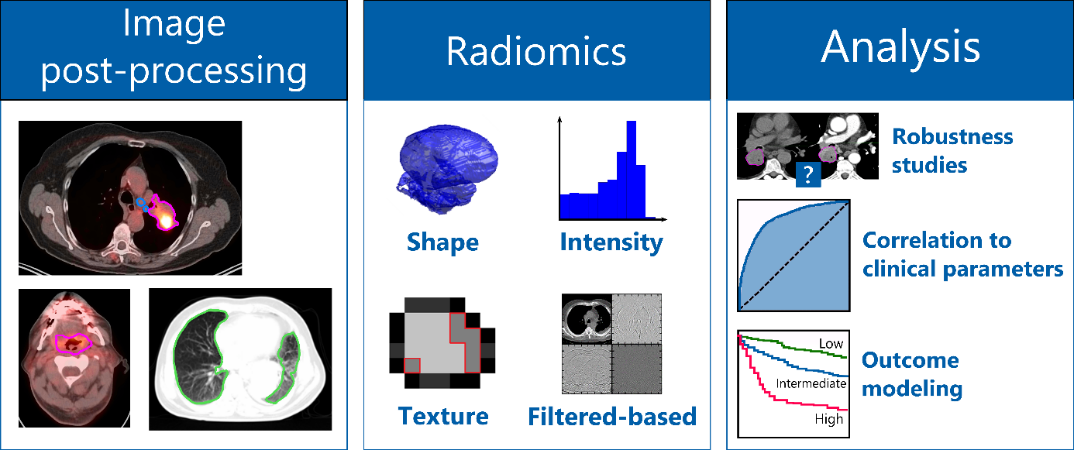
Our group is located in the radiation therapy department at the University Hospital of Zurich (USZ) which allows us to work in a highly interdisciplinary setting with the medical doctors and radiation biologists to address clinical needs. Our research focuses on:
- the robustness of radiomic features against scanning and imaging uncertainties
- the correlation of radiomic features to clinical parameters or patient outcome modeling to stratify patients into different risk groups for different tumor types (such as brain, head and neck, melanoma and lung)
- the correlation of radiomic features with tumor biology (such Gleason score or Human Papillomavirus)
- the translation of preclinical radiomic signatures to clinical setting
- the repetitive tumor and organs art risk monitoring using the concept of delta radiomics (time variation in radiomic features) and recently installed MRI-Linac
- the implementation of deep learning concepts for medical image analysis and outcome modeling
- Privacy preserving distributed learning for outcome modelling
Selection of publications
- Vuong D, Tanadini-Lang S, Huellner MW, Veit-Haibach P, Unkelbach J, Andratschke N, Kraft J, Guckenberger M, Bogowicz M. Interchangeability of radiomic features between [18F]-FDG PET/CT and [18F]-FDG PET/MR. Med Phys. 2019 Apr;46(4):1677-1685
- Tanadini-Lang S, Bogowicz M, Veit-Haibach P, et al. Exploratory Radiomics in Computed Tomography Perfusion of Prostate Cancer. Anticancer research. 2018;38(2):685-690.
- Pavic M, Bogowicz M, Würms X, et al. Influence of inter-observer delineation variability on radiomics stability in different tumor sites. Acta Oncologica. 2018:1-5.
- Bogowicz M, Riesterer O, Stark LS, et al. Comparison of PET and CT radiomics for prediction of local tumor control in head and neck squamous cell carcinoma. Acta Oncol. 2017;56(11):1531-1536.
- Bogowicz M, Riesterer O, Ikenberg K, et al. Computed Tomography Radiomics Predicts HPV Status and Local Tumor Control After Definitive Radiochemotherapy in Head and Neck Squamous Cell Carcinoma. International journal of radiation oncology, biology, physics. 2017;99(4):921-928.
- Bogowicz M, Leijenaar RTH, Tanadini-Lang S, et al. Post-radiochemotherapy PET radiomics in head and neck cancer – The influence of radiomics implementation on the reproducibility of local control tumor models. Radiother Oncol. 2017;125(3):385-391.
- Nesteruk M, Lang S, Veit-Haibach P, et al. Tumor stage, tumor site and HPV dependent correlation of perfusion CT parameters and [18F]-FDG uptake in head and neck squamous cell carcinoma. Radiotherapy and Oncology. 2015;117(1):125-131.
Zusammenfassung für Patientinnen und Patienten:
Trotz grosser Fortschritte in der Immuntherapie sprechen leider nicht alle Patientinnen und Patienten auf eine solche Behandlung an und sind dennoch deren Nebenwirkungen ausgesetzt. In diesem Forschungsprojekt, das die Klinik für Radio-Onkologie zusammen mit der Dermatologie durchführt, möchten wir herausfinden, welche Patientinnen und Patienten mit schwarzem Hautkrebs (malignes Melanom) tatsächlich von einer Immuntherapie profitieren: hierfür analysieren wir die Patientinnen- und Patienten-individuellen Eigenschaften des Hautkrebs, wie er sich auf Computertomographie und PET-Aufnahmen präsentiert, und hoffen so bereits vor Therapiebeginn das zukünftige Ansprechen vorhersagen zu können.
Projet title:
Immuno-Radiomics as a novel prognostic and predictive biomarker in malignant melanoma treated with immune checkpoint inhibition – a multi-systems approach
Principle investigators:
Matthias Guckenberger, Department for Radiation Oncology, USZ
Mitchell Levesque, Department of Dermatology, USZ
Contributing researchers:
L. Basler, M. Bogowicz, R. Dummer, H. Gabrys, S. Hogan, R. Förster, S. Tanadini-Lang, Diem Vuong,
Funding:
This project is supported by the Cancer Research Center: 2018 – 2020
Background:
Despite immune checkpoint inhibitors (ICI) having improved overall survival in metastatic melanoma patients substantially, more than 50% of all patients do not respond to treatment. Identifying predictive biomarkers for the response to ICI is challenging and so far only PD-L1 expression is established clinically, which may however be insufficient for patient selection. Non-invasive anatomical and functional imaging using CT, MRI and PET is performed routinely and repetitively during the treatment course, providing the opportunity for continuous response evaluation and/or prediction. This translational and interdisciplinary research project aims to identify prognostic and predictive imaging biomarkers for treatment with immune checkpoint inhibitors in metastatic melanoma patients. Imaging biomarkers are based on comprehensive statistical and mathematical characterization of medical images (radiomics). We combine the expertise of the Levesque lab in identifying predictive molecular biomarkers for immunotherapy response in metastatic melanoma (Krieg et al., 2018) with the expertise of the group the Guckenberger & Tanadini-Lang group in quantitative radiomic biomarker research (Bogowicz et al., 2016).
Aims:
To establish a multi-systems prognostic and prediction model of response to immunotherapy in patients with metastatic melanoma. The project will focus on evaluation of CT and FDG-PET imaging biomarkes – radiomics – and the integration of radiomics biomarkers with biospecimen biomarkers.
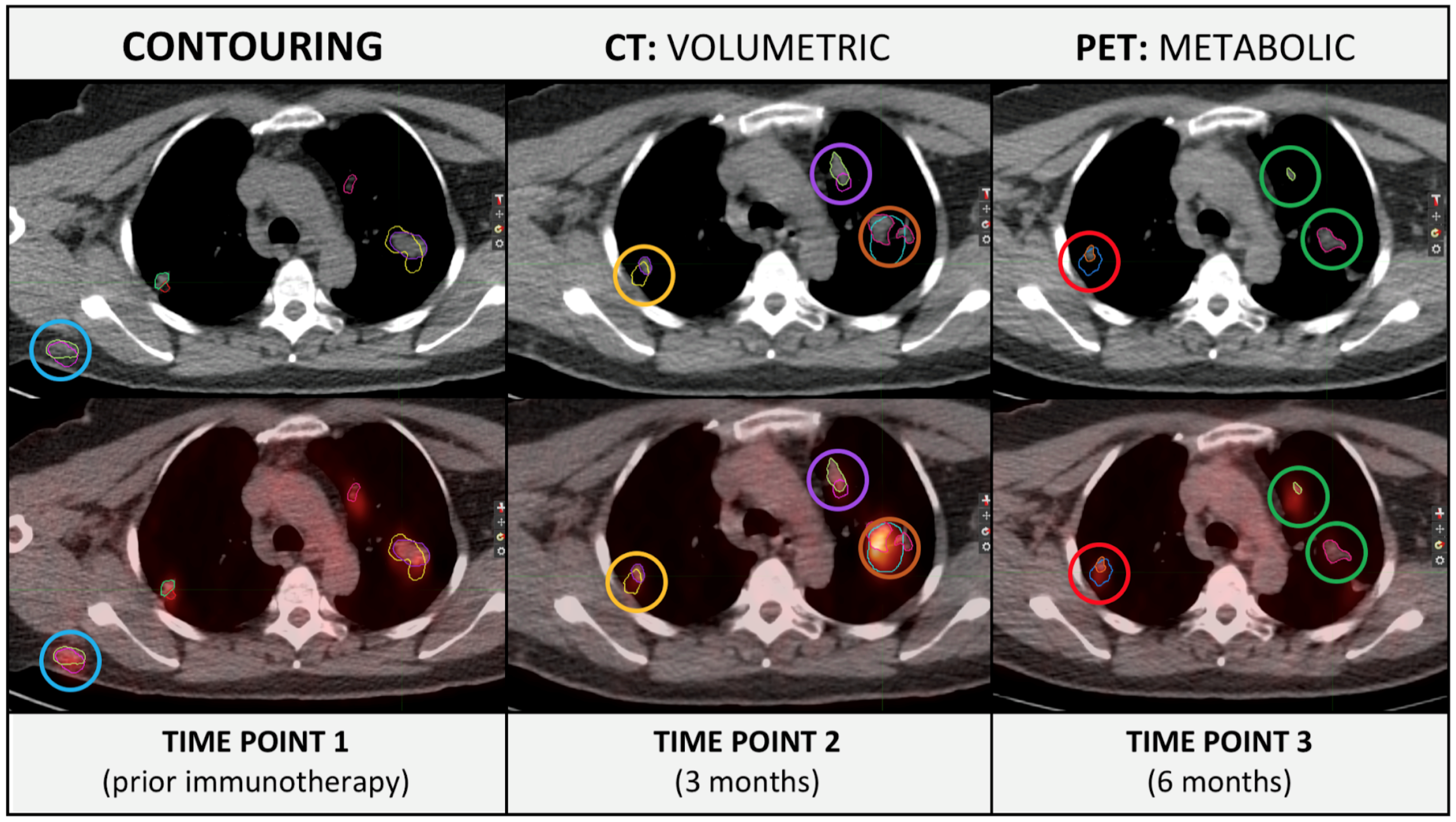
Research Output:
ASCO Abstract 2019 (submitted):
- Delta-radiomics for prediction of pseudoprogression in malignant melanoma treated with immune checkpoint inhibition. Lucas Basler, Hubert Gabrys, Sabrina A. Hogan, Marta Bogowicz, Diem Vuong, Stephanie Tanadini-Lang, Robert Foerster, Martin Hüllner, Reinhard Dummer, Matthias Guckenberger, Mitchell P. Levesque
- Major finding: Metastasis location significantly influenced response rates and CT-based delta-radiomics is a promising biomarker for early differentiation between pseudoprogression and true progression in metastatic melanoma patients treated with ICI.
Zusammenfassung für Patientinnen und Patienten:
Die personalisierte Medizin hat das Ziel, jedem Patienten und jeder Patientin eine genau auf ihn oder sie abgestimmte Therapie anzubieten. Dazu muss zunächst das Krankheitsbild der Patientinnen und Patienten sehr genau charakterisiert werden. Häufig wird dies gemacht, indem aus Tumorbiopsien genetische Marker bestimmt werden. In diesem Projekt wollen wir medizinische Bildgebungsmethoden (CT, MR) nutzen, um das Krankheitsbild der Patientinnen und Patienten mit mathematischen Methoden zu beschreiben. Dieses Projekt ist ein Kollaborationsprojekt mit dem Universitätsspital Basel, dem Inselspital Bern, dem CHUV in Lausanne und dem Kantonsspital Tessin.
Projet title:
Project Plan: Radiomics for comprehensive patient and disease phenotyping in personalized health (IMAGINE)
Principle investigators:
Matthias Guckenberger, Department for Radiation Oncology, USZ
Contributing researchers:
Bram Stieltjes, University Hospital Basel
Maricio Reyes, University of Bern
Roland Wiest, University Hospital Bern
Christoph Stippich, University Hospital Zürich
Nicolaus Andratschke, University Hospital Zürich
Reto Meuli, CHUV Lausanne
Cornelia Kruschel Weber, University Hospital Zürich
Stephanie Tanadini-Lang, University Hospital Zürich
Adrien Depeursinge, HES-SO Valais
Olivier Michielin, CHUV Lausanne
Giorgio Treglia, Ente Ospedaliero Cantonale
Funding:
This project is supported by the Swiss Personalised Health Network (SPHN): 2019 – 2021
Background
Early personalized health initiatives and projects have uni-dimensionally focused on biological sample-based disease phenotyping. However, environmental factors are known to attenuate the association between the patients` genetic background and outcome. Consequently, the need for analysis of “downstream” phenotypes has been recognized and has become a strong field of research within personalized health. In this context, multi-modal medical imaging is an important source of phenotype information and plays a prominent role in the diagnosis, staging, and treatment response monitoring. Despite the fact that imaging is standard practice to characterize the patient individual disease and to estimate patient individual outcome, the incorporation of medical images into personalized health has been blocked by the current standard practice of image analysis: image analysis is mostly a manual process resulting in predominantly qualitative image characterization. To unlock the full potential of medical images for personalized health, quantitative image characterization (Radiomics) has become a highly promising field of research.
Aims:
In this driver project, we propose an initiative to build a Swiss-wide infrastructure for image-based biomarker research & analysis. We will standardize imaging, image analysis and image-based outcome modelling to evaluate the value of MR images acquired in clinical routine as prognostic and predictive biomarker in patients treated for Glioblastoma multiforme. We hypothesize that radiomic biomarkers will improve outcome modelling and improve selection of the most appropriate patient-individual treatment strategy.
Zusammenfassung für Patientinnen und Patienten:
Neue zielgerichtete Medikamente und die Immuntherapie haben heute bei vielen Krebspatientinnen und -patienten mit einer metastasierten Erkrankung die Prognose verbessern können. Leider entwickeln dennoch die Mehrzahl der Patientinnen und Patienten eine Resistenz gegen diese Medikamente, d.h. der Krebs verändert sich, die Medikamente verlieren ihre Wirkung und die Erkrankung schreitet voran. Wenn diese Veränderung der Krebserkrankung und das Wachsen des Krebs nur an auf eine oder wenigen Stellen im Körper beschränkt ist (Oligometastasierung), dann können diese entarteten Tumorherde heute mittels moderner fokussierter Bestrahlung (eine sogenannte stereotaktische Bestrahlung) abgetötet werden. Diese internationale Studie untersucht daher, wie fokussierte Bestrahlung und zielgerichtete Medikamente optimal und auch sicher kombiniert werden können.
Projet title:
TOaSTT: Prospective registry study to evaluate toxicity and efficacy of concomitant systemic molecularly targeted and/or immunotherapy and stereotactic radiation therapy for locally recurrent and/or metastatic cancer
Principle investigators:
Prof. Matthias Guckenberger, Department for Radiation Oncology, USZ
Prof Anca Grosu (Germany), Universitätsklinikum Freiburg
Contributing researchers:
This project is conduced within the DEGRO working group Stereotactic Radiotherapy & Radiosurgery
Dr. Indira Madani, Ph.D., Dr. Stephanie Kroeze, Ph.D., Dr. Corinna Fritz, Dr. Jana Heitmann
Funding:
This project is supported by Varian: 2016 – 2020
Background:
Cancer is one of the leading causes of death worldwide, where cancer progression and/or recurrence are the primary cause of cancer-related mortality. With advances in cancer care including new anti-cancer therapies (e.g., molecularly targeted therapy, immunotherapy, stereotactic radiotherapy [SRT]) the goal of treatment of locoregionally recurrent and/or metastatic cancer has been shifting from palliation to personalized disease control and survival. However, knowledge of safety and efficacy of such combined treatments including latest systemic drugs and radiotherapy, especially SRT, is lacking. Prospective safety evaluation of SRT and molecularly targeted therapy and/or immunotherapy in the form of traditional interventional clinical trials is extremely difficult for all possible permutations of treatment options and practices considering the different cancer types, different lines of previous treatments, different anatomical locations, volume and number of irradiated target volumes. In the absence of prospective evidence, institutions may therefore fail to gain relevant experience independently due to this heterogeneity.
Aims:
We therefore propose to establish an international, multi-center prospective registry to evaluate treatment-induced toxicity and efficacy of concomitant molecularly targeted therapy and/or immunotherapy and SRT for locoregionally recurrent and/or metastatic cancer.
Research Output:
ESTRO Abstracts 2019 (submitted):
Cumulative metastases volume and not number of metastases predicts survival in melanoma patients treated with stereotactic radiotherapy or radiosurgery: J. Heitmann, S.G.C. Kroeze, O. Blanck, S. Stera, K.K. Henning, F. Roeder, S. Combs, D. Kaul, J.J.C. Verhoeff, M. Schymalla, A.L. Grosu, F. Eckert, F. Lohaus, N. Abassi-Senger, G. Henke, M. Szuecs, M. Geier, P. Ost, D. Buergy, M. Guckenberger
Major finding:
For melanoma patients with brain metastases treated with concurrent targeted- or immunotherapy and SRT without concurrent WBRT, irradiated brain metastases volume impacts OS but not the number of treated brain metastases. We propose adapting the molGPA score score for melanoma patients treated with SRT and concurrent targeted- or immunotherapy.
Safety and efficacy of concurrent SRT and targeted- or immunotherapy for melanoma brain metastases: J. Heitmann, S.G.C. Kroeze, O. Blanck, D. Imhoff, K.K. Henning, F. Roeder, S. Combs, D. Kaul, J.J.C. Verhoeff, M. Schymalla, A.L. Grosu, F. Eckert, F. Lohaus, N. Abassi-Senger, G. Henke, M. Szuecs, M. Geier, P. Ost, D. Buergy, M. Guckenberger
Major finding:
Concurrent immune- or targeted therapy with SRT for melanoma brain metastases appears safe in this multicenter analysis. Longest OS was observed when SRT was combined with immune checkpoint inhibition; use of steroids should be considered carefully as this was associated with worse OS.
Stereotactic radiotherapy concurrent to targeted- or immunotherapy for oligometastatic NSCLC: clinical scenarios affecting prognosis: S.G.C. Kroeze, C. Fritz, D. Kaul, O. Blanck, K.H. Kahl, F. Roeder, S. Siva, J.J.C. Verhoeff, A.L. Grosu, M. Schymalla, M. Glatzer, M. Szuecs, M. Geier, G. Skazikis, I. Sackerer, F. Lohaus, F. Eckert, M. Guckenberger
Major finding:
Patients treated with concurrent immuno- or targeted therapy and SRT of oligometastases showed a favorable survival with acceptable toxicity. SRT allowed continuation of targeted-, or immunotherapy for minimum 1 year in a relevant number of patients. These observations need to be further evaluated within prospective trials.
Zusammenfassung für Patientinnen und Patienten:
Trotz grosser Fortschritte in der Immuntherapie des Malignen Melanoms, des schwarzen Hautkrebs, sprechen leider nicht alle Patientinnen sowie Patienten auf eine solche Behandlung an und sind dennoch deren Nebenwirkungen ausgesetzt. In diesem Forschungsprojekt, das vom Schweizer Nationalfonds gefördert wird, möchten Forscherinnen und Forscher aus verschiedenen Institutionen und mit verschiedenen, komplementären Techniken und Kompetenzen besser verstehen, welche Patientinnen und Patienten auf eine Immuntherapie ansprechen werden und welche nicht. Methoden des maschinellen Lernens sollen angewendet werden, um die grossen und komplexen Datenmengen integrieren und auswerten zu können. Damit möchten wir in Zukunft genauer vorhersagen können, wie wir jeden einzelnen Patienten sowie jede einzelne Patientin optimal und individuell behandeln können.
Project title:
Machine learning based integration of high-dimensional cytometry and radiomics data to identify and monitor cancer-immunotherapy responses
Principle investigators:
Prof. Burkhard Becher, Institut für Experimentelle Immunologie, UZH
Prof. Manfred Claassen, Institut für Molekulare Systembiologie ETH Zürich
Prof. Matthias Guckenberger, Department for Radiation Oncology, USZ
Prof. Mitchell Levesque, Department of Dermatology, USZ
Funding:
Schweizerischer Nationalfonds (SNF) Sinergia: 2019 – 2022
Background:
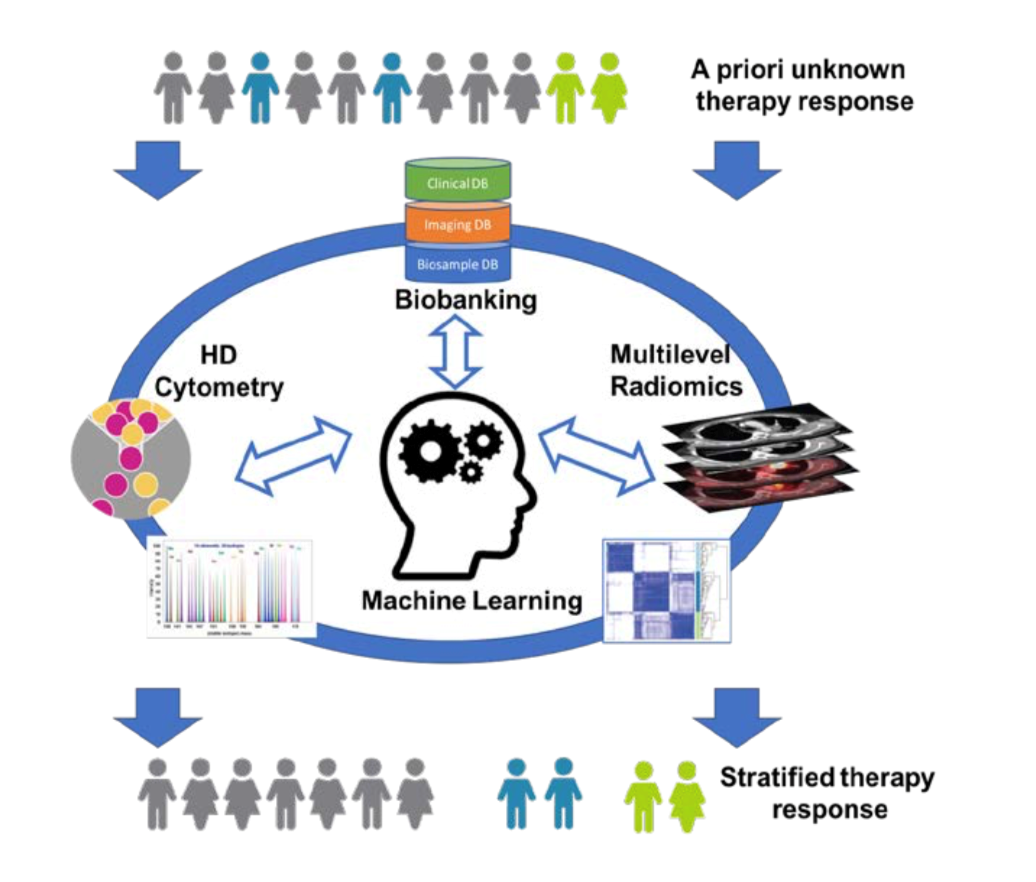
Immunotherapy has revolutionized the way cancer patients are treated today and has revived efforts to seek therapeutic targets to raise anti-tumor immunity across all malignant entities. Check-point inhibition (CPI), CAR-T cells, and cytokines are combined with conventional approaches to treat cancer and to elicit anti-tumor immunity. Measuring the successes of clinical trials often requires monitoring through general clinical parameters, and the outcome measurement is often the rate of progression based on radiological imaging and survival. The downside of this conventional monitoring approach is that patient responses are often detected long after treatment initiation and therapy-resistant patients incur the adverse effects while delaying the administration of potentially better-tailored therapies. Biomarkers that predict and monitor patient responses to immunotherapy are only now emerging for some immunotherapies; whereas new therapeutic approaches often still rely on conventional clinical monitoring.
While biased analysis of blood parameters, and even targeted cytometry and bulk-omics have yielded some insights into potential biomarkers, high-dimensional single-cell cytometry, combined with unbiased algorithm-based pattern recognition have revolutionized biomarker identification and personalized medicine. Similarly, advances in radiological imaging and in particular quantitative radiological image analysis (radiomics) now allow for a more precise determination of tumor burden, cancer characterization and assessment of treatment response.
Aims:
This project will address malignant melanoma patients undergoing immune checkpoint inhibition (aPD-1 or aPD1/aCTLA-4): we will seek to i) identify biomarkers to predict the therapeutic response to combination cancer immunotherapy, ii) monitor therapy responses during combination therapy and iii) analyze polymorphonuclear granulocytes as indicators of anti-tumor immunity. The project combines cutting-edge clinical oncology and computational radiological image analysis and innovative computational biology to identify prognostic and predictive signatures to establish clinically applicable tools to guide precision medicine decisions and to identify new therapeutic targets for the treatment of cancer.
Zusammenfassung für Patientinnen und Patienten:
Viele Tumore breiten sich über die Lymphwege aus und bilden Metastasen in benachbarten Lymphknoten. Moderne medizinische Bildgebungsverfahren sind heute das wichtigste Werkzeug um Lymphknotenmetastasen zu diagnostizieren. Das beinhaltet die Computertomographie (CT), Magnetresonanztomographie (MRT) und die Positronenemissionstomographie (PET). Allerdings können mit den heutigen Verfahren nur Lymphknotenmetastasen detektiert werden, welche bereits eine gewisse Grösse erreicht haben. Mikroskopische Ausbreitungen des Tumors können nicht dargestellt werden. Die Behandlung dieser Tumore stellt daher eine grosse Herausforderung dar. Krebspatienten mit Tumoren im Kopf-Hals Bereich können oftmals mit einer Radiochemotherapie geheilt werden. Allerdings ist die Behandlung in den meisten Fällen mit erheblichen Nebenwirkungen verbunden. Mundtrockenheit, Schluckbeschwerden und damit verbundene Folgeerscheinungen mindern die Lebensqualität vieler Patienten, die ihre Krebserkrankung überlebt haben. Diese Nebenwirkungen entstehen zum Teil dadurch, dass die Ausbreitung des Tumors nicht genau bekannt ist. Daher werden grosse Teile des Halses prophylaktisch bestrahlt. Um Nebenwirkungen zu reduzieren ist es nötig genauer vorauszusagen welche Teile des Lymphabflussgebiets mit hoher Wahrscheinlichkeit bereits vom Tumor infiltriert sind. Zu diesem Zweck entwickeln wir verbesserte statistische Modelle um die lymphatische Ausbreitung des Tumors zu beschreiben. Die Modelle werden benutzt um die Strahlentherapie dem individuellen Patienten anzupassen, mit dem Ziel Nebenwirkung zu reduzieren.
Project title:
Artificial Intelligence based modelling of lymphatic cancer progression
Principal investigator:
Jan Unkelbach, Department for Radiation Oncology, USZ
Contributing researchers:
Roman Ludwig, Bertrand Pouymayou, Panagiotis Balermpas
International collaborators:
Vincent Grégoire, Centre Léon Bérard, France; Roland Giger, Inselspital Bern, CH; Oliver Riesterer, Kantonsspital Aarau, CH
Funding:
This project is supported by the Clinical Research Priority Program Artificial Intelligence in Oncological Imaging: 2019 – 2025
Background:
Research in medical physics has provided tremendous improvements to the technology used to treat cancer patients with radiotherapy. This includes the development of intensity-modulated radiotherapy (IMRT), proton therapy, and image-guided radiotherapy (IGRT). These technologies allow us to deliver highly conformal radiation dose distributions to tumors and spare adjacent healthy tissues from radiation. With these technological improvements in precision, the biggest source of uncertainty today is often the definition of the target volume, i.e. the region to be irradiated. Modern imaging techniques like Magnetic Resonance Imaging (MRI) and Positron Emission Tomography (PET) improved the definition of the gross tumor volume (GTV). In contrast, definition of the clinical target volume (CTV) remains very challenging. The CTV includes possible microscopic extension of the tumor, i.e. tissues that are at risk of harboring tumor cells even though these cannot be detected with current imaging modalities. CTV definition is therefore not based on imaging the tumor per se, but rather on understanding the anatomically defined routes of loco-regional tumor progression. Many major tumors types spread locally through the lymphatic system and form metastasis in regional lymph nodes. Therefore, the CTV includes lymph node levels that are at risk of harboring tumor cells even though tumor cannot be detected with today’s imaging modalities. The concept is called elective lymph node irradiation.
This presentation, given remotely at the annual ESTRO meeting 2020, provides an overview on our work on computational methods to improve or automate the definition of the clinical target volume (CTV).
Aims:
The goal of this project is to personalize and improve the definition of the elective CTV for head & neck squamous cell carcinoma (HNSCC) patients based on the individual patient’s state of clinical tumor progression. We develop a statistical model of lymphatic tumor spread based on the methodology of Bayesian networks and Hidden Markov Models to better quantify the risk of occult metastases in the different lymph node levels in the neck (see illustration in figure 1). In this model, each lymph node level is associated with a binary random variable for the microscopic state, which indicates whether or not the level truly harbors tumor including occult metastases. The microscopic state is a latent state that is not directly observable for radiotherapy patients. Associated with the microscopic state is an observable macroscopic state, which indicates whether a lymph node level contains visible metastases based on PET-CT imaging (and possible additional diagnostic modalities). Macroscopic and microscopic states are linked via the specificity and sensitivity of PET-CT imaging. The microscopic states are connected via directed arcs if one lymph node level receives efferent lymphatics from the other. Each arc is associated with a probability of tumors to spread from one level to the next. For example, level II and level III are connected by an arc reflecting the directional lymph flow from level II to level III, which allows tumors to spread from level II further down to level III. Thus, the graph of the Bayesian network reflects the anatomy of the lymphatic drainage system.
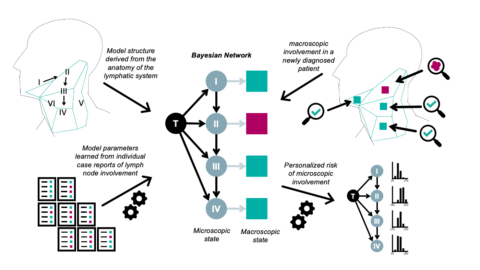
Figure 1: Schematic illustration of model of lymphatic progression.
The parameters of the model, the probabilities of tumor cells to spread from the primary tumor site to the lymph node levels and in between lymph node levels, can be learned from datasets of lymphatic progression patterns. To that end, we develop the online platform LyProx to host, share and visualize datasets on patterns of lymph node involvement in HNSCC patients, which is the basis for a better quantification of tumor progression. The GUI allows users to study how the frequency of lymph node metastases in a lymph node level depends on location and stage of the primary tumor and the presence of lymph node metastases in other lymph node levels (figure 2).
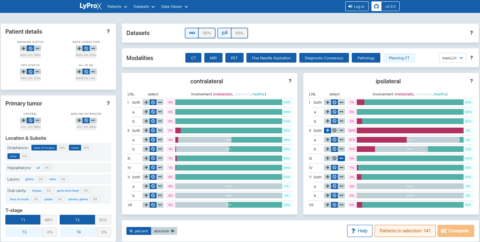
Figure 2: GUI of LyProx.
Publications:
- B. Pouymayou, P. Balermpas, O. Riesterer, M. Guckenberger, and J. Unkelbach, “A Bayesian network model of lymphatic tumor progression for personalized elective CTV definition in head and neck cancers,” Phys Med Biol, vol. 64, no. 16, p. 165003, 14 2019, doi: 10.1088/1361-6560/ab2a18.
- R. Ludwig, B. Pouymayou, P. Balermpas, and J. Unkelbach, “A hidden Markov model for lymphatic tumor progression in the head and neck,” Sci Rep, vol. 11, no. 1, p. 12261, Jun. 2021, doi: 10.1038/s41598-021-91544-1.
- R. Ludwig et al., “Detailed patient-individual reporting of lymph node involvement in oropharyngeal squamous cell carcinoma with an online interface,” Radiother Oncol, vol. 169, pp. 1–7, Apr. 2022, doi: 10.1016/j.radonc.2022.01.035.
- R. Ludwig et al., “A dataset on patient-individual lymph node involvement in oropharyngeal squamous cell carcinoma,” Data Brief, vol. 43, p. 108345, Aug. 2022, doi: 10.1016/j.dib.2022.108345.
- J. Unkelbach et al., “The role of computational methods for automating and improving clinical target volume definition,” Radiother Oncol, vol. 153, pp. 15–25, Dec. 2020, doi: 10.1016/j.radonc.2020.10.002.
Zusammenfassung für Patientinnen und Patienten:
Die Protonentherapie ist eine spezielle Form der Strahlentherapie bei der der Patient mit Protonen bestrahlt wird, anstelle der sonst üblichen Röntgenstrahlung. Die Protonenbestrahlung bietet den Vorteil, dass die Strahlendosis im gesunden Gewebe, welches den Tumor umgibt, reduziert wird. Allerdings ist die Protonentherapie technisch aufwendig und mit höheren Kosten verbunden als die konventionelle Röntgentherapie. Daher erfolgen heute nur etwa 1% der Bestrahlungen weltweit mit Protonen. Das Paul Scherer Institut in Villingen ist zur Zeit die einzige Institution in der Schweiz die Protonentherapie anbietet. Folglich können nicht alle Patienten, die potentiell von der Protonenbestrahlung profitieren könnten, auch mit Protonen behandelt werden. Daher müssen Konzepte entwickelt werden um den Nutzen der Protonentherapie für den einzelnen Patienten sowie die Gesamtheit der Krebspatienten zu optimieren. Die zugrundeliegende Hypothese dieses Projekts ist es, dass kombinierte Protonen-Photonen-Behandlungen, bei denen ein Teil der Bestrahlung mit Protonen erfolgt und der Rest mit Röntgenstrahlung, dazu nötig sind. Wir untersuchen in diesem Projekt zwei Fragestellungen: Erstens, wie können Protonen und Röntgenstrahlung optimal kombiniert werden um die Bestrahlung eines individuellen Patienten zu optimieren? Zweitens, wie sollen die verfügbaren Kapazitäten in der Protonentherapie optimal über das Patientenkollektiv verteilt werden, d.h. wie viele Protonenfraktionen soll jeder Patient erhalten? Dazu entwickeln wir neue mathematische Algorithmen für die Bestrahlungsplanung, welche die Kombination vor Protonen und Röntgenstrahlung optimieren.
Project title:
Combined proton-photon radiotherapy
Principle investigators:
Jan Unkelbach, Department for Radiation Oncology, USZ
Contributing researchers:
Louise Marc, Silvia Fabiano, Florian Amstutz (PSI), Tony Lomax (PSI), Christian Richter (Oncoray Dresden)
Funding:
This project is supported by the Swiss Cancer League: 2019 – 2023
Background:
Proton therapy is widely considered a superior form of radiotherapy compared to conventional X-ray based radiotherapy. The main advantage of proton therapy is the reduction of integral dose to normal tissues surrounding the tumor, which may reduce radiation induced side effects and secondary cancer risk for selected patients. However, there are two main disadvantages of proton therapy: 1) protons are more susceptible to organ motion and range uncertainties so that the conformity of the dose distribution is not always superior to X-rays, and 2) proton therapy is more costly and less available. Currently, proton therapy accounts for less that 1% of all radiotherapy treatments worldwide and is offered by only one institution in Switzerland. Therefore, not all patients who would potentially benefit from proton therapy can be treated with protons. Both at the level of the individual patient as well as the population of cancer patients as a whole, the question is how to optimally make use of proton therapy. We hypothesize that optimized combinations of proton treatments and conventional X-ray treatments are needed to address this question.
A presentation of our work on combined proton-photon radiotherapy, given at the AAPM annual meeting in 2021, can be found here.
Aims:
- An individual patient may benefit from an optimized combination of protons and X-rays, which optimally exploits the advantages of both radiation modalities. Hence, for a patient at hand, the ratio of tumor dose and normal tissue dose may be improved, which in turn may allow for improved chances of cure or reduced side effects. In this project, we will develop novel mathematical algorithms for radiotherapy treatment planning that yield the optimal combination of protons and X-rays.
- As proton therapy is more costly and less available, efforts must be made to optimally make use of this limited resource. This is especially important in the context of ever increasing health care costs. In this project, we will therefore develop algorithms to optimize the allocation of proton therapy slots over a patient cohort, in order to optimize the benefit of proton therapy at the population level.
- Combined proton-photon treatments may play a role in developing new cost-effective concepts for proton therapy. High proton therapy costs are mostly due to the Gantry used to rotate the treatment beam around the patient. Costs could be reduced substantially by using a fixed horizontal proton beamline. This would allow for a much more widespread application of proton therapy in many more hospitals. The treatment planning algorithms developed in this project will allow us to investigate the potential of the following concept: A horizontal proton beamline integrated in a conventional treatment room equipped with a standard Linac for X-ray therapy, and a standard treatment table to irradiate the patient in lying position.
Publications:
- S. Fabiano, P. Balermpas, M. Guckenberger, and J. Unkelbach, “Combined proton–photon treatments – A new approach to proton therapy without a gantry,” Radiotherapy and Oncology, vol. 145, pp. 81–87, 2020.
- S. Fabiano, M. Bangert, M. Guckenberger, and J. Unkelbach, “Accounting for Range Uncertainties in the Optimization of Combined Proton-Photon Treatments Via Stochastic Optimization,” Int. J. Radiat. Oncol. Biol. Phys., vol. 108, no. 3, pp. 792–801, Nov. 2020, doi: 10.1016/j.ijrobp.2020.04.029.
- N. Loizeau et al., “Optimal Allocation of Proton Therapy Slots in Combined Proton-Photon Radiation Therapy,” Int J Radiat Oncol Biol Phys, vol. 111, no. 1, pp. 196–207, Sep. 2021, doi: 10.1016/j.ijrobp.2021.03.054.
- J. Unkelbach, M. Bangert, K. De Amorim Bernstein, N. Andratschke, and M. Guckenberger, “Optimization of combined proton-photon treatments,” Radiother Oncol, vol. 128, no. 1, pp. 133–138, Jul. 2018, doi: 10.1016/j.radonc.2017.12.031.
- S. Fabiano, N. Torelli, D. Papp, and J. Unkelbach, “A novel stochastic optimization method for handling misalignments of proton and photon doses in combined treatments,” Phys Med Biol, vol. 67, no. 18, Sep. 2022, doi: 10.1088/1361-6560/ac858f.
- L. Marc, S. Fabiano, N. Wahl, C. Linsenmeier, A. J. Lomax, and J. Unkelbach, “Combined proton-photon treatment for breast cancer,” Phys Med Biol, vol. 66, no. 23, Nov. 2021, doi: 10.1088/1361-6560/ac36a3.
- F. Amstutz et al., “Combined proton-photon therapy for non-small cell lung cancer,” Med Phys, vol. 49, no. 8, pp. 5374–5386, Aug. 2022, doi: 10.1002/mp.15715.
- D. Papp and J. Unkelbach, “Technical note: Optimal allocation of limited proton therapy resources using model-based patient selection,” Med Phys, vol. 49, no. 8, pp. 4980–4987, Aug. 2022, doi: 10.1002/mp.15812.
Zusammenfassung für Patientinnen und Patienten:
In der Strahlentherapie geht es oft darum, den Tumor möglichst genau zu bestrahlen und gleichzeitig die Strahlendosis im gesunden Gewebe, welches den Tumor umgibt, zu minimieren. Dazu muss entschieden werden, aus welchen Richtungen der Patient bestrahlt wird und mit welchen Intensitäten. Die Strahlungsfelder aus verschiedenen Richtungen müssen sich im Tumor so überlagern, dass eine ausreichend hohe Dosis appliziert wird. Gleichzeitig müssen besonders strahlenempfindliche Organe vermieden werden. Heutzutage nutzt man mathematische Optimierungsalgorithmen, um die optimalen Strahlungsfelder zu bestimmen. In unserer Klinik arbeiten wir an der Verbesserung und Weiterentwicklung dieser Algorithmen.
Project title:
Treatment plan optimization for intensity-modulated radiotherapy (IMRT/IMPT)
Principle investigator:
Jan Unkelbach, Department for Radiation Oncology, USZ
Contributing researchers:
Nathan Torelli, Louise Marc, Silvia Fabiano, David Papp (North Carolina State University, USA)
Background:
Treatment planning for radiotherapy is based on two main components: Dose calculation algorithms and mathematical optimization algorithms. Dose calculation algorithms use physical models to describe the interaction of radiation in tissue to calculate the distribution of absorbed radiation dose in the patient. Mathematical optimization methods are used to optimize intensities and incident directions of external radiation fields to irradiate the tumor while minimizing the radiation dose to surrounding normal tissues. Our group has worked on many problems related to the further development of optimization algorithms for treatment planning.
Aims:
Our main projects in the field of treatment plan optimization are:
- In the context of our project on combined proton-photon radiotherapy, we work on treatment planning algorithms to simultaneously optimize IMRT and IMPT dose distributions to determine the optimal combination of both modalities.
- In the context of our project on spatiotemporal fractionation, we work on treatment planning algorithms based on biologically effective dose (BED) instead of physical dose. This allows the simultaneous optimization of multiple distinct dose distributions for different fractions.
- In the context of both projects, we work on the development of novel robust optimization techniques, which incorporate uncertainty in the IMRT/IMPT optimization problem.
- We work on treatment planning methods for patients with high metastatic tumor burden in whom it is not possible to deliver a curative radiation dose to all lesions without exceeding normal tissue constraints. Here, we work on cell-kill based objective functions to optimally distribute the radiation dose over all lesions.
- Building on algorithms for multileaf collimator leaf sequencing and direct aperture optimization, we work on methods and tools to create deliverable treatments plans in in-house research software. We further work on tools to import these treatment plans into the commercial treatment planning system Eclipse to allow clinical translation of our research.
Publications:
- S. Fabiano, N. Torelli, D. Papp, and J. Unkelbach, “A novel stochastic optimization method for handling misalignments of proton and photon doses in combined treatments,” Phys Med Biol, vol. 67, no. 18, Sep. 2022, doi: 10.1088/1361-6560/ac858f.
- S. Fabiano, M. Bangert, M. Guckenberger, and J. Unkelbach, “Accounting for Range Uncertainties in the Optimization of Combined Proton-Photon Treatments Via Stochastic Optimization,” Int. J. Radiat. Oncol. Biol. Phys., vol. 108, no. 3, pp. 792–801, Nov. 2020, doi: 10.1016/j.ijrobp.2020.04.029.
- L. Marc, S. Fabiano, N. Wahl, C. Linsenmeier, A. J. Lomax, and J. Unkelbach, “Combined proton-photon treatment for breast cancer,” Phys Med Biol, vol. 66, no. 23, Nov. 2021, doi: 10.1088/1361-6560/ac36a3.
- J. Unkelbach, M. Bangert, K. De Amorim Bernstein, N. Andratschke, and M. Guckenberger, “Optimization of combined proton-photon treatments,” Radiother Oncol, vol. 128, no. 1, pp. 133–138, Jul. 2018, doi: 10.1016/j.radonc.2017.12.031.
- J. Unkelbach, D. Papp, M. R. Gaddy, N. Andratschke, T. Hong, and M. Guckenberger, “Spatiotemporal fractionation schemes for liver stereotactic body radiotherapy,” Radiother Oncol, vol. 125, no. 2, pp. 357–364, Nov. 2017, doi: 10.1016/j.radonc.2017.09.003.
- J. Unkelbach et al., “Robust radiotherapy planning,” Physics in Medicine & Biology, vol. 63, no. 22, p. 22TR02, 2018.
Prior work we build on:
- A. Cassioli and J. Unkelbach, “Aperture shape optimization for IMRT treatment planning,” Physics in medicine and biology, vol. 58, no. 2, pp. 301–18, Jan. 2013, doi: 10.1088/0031-9155/58/2/301.
Zusammenfassung für Patientinnen und Patienten:
Die Bestrahlung von Tumoren erfolgt in der Regel fraktioniert, d.h. der Patient wird über einen Zeitraum von mehreren Tagen oder Wochen täglich bestrahlt. In der heutigen Praxis wird die Strahlendosis gleichmässig auf die Behandlungstage aufgeteilt und der Tumor wird jeden Tag mit der gleichen Dosis bestrahlt. Das Konzept der raum-zeitlichen Fraktionierung, welches in diesem Projekt weiter untersucht wird, stellt diese Praxis in Frage. In vorangegangenen Arbeiten wurde gezeigt, dass es potentiell von Vorteil sein kann an unterschiedlichen Tagen verschiedene Dosisverteilungen zu applizieren. Dabei ist es das Ziel die Bestrahlungen so zu gestalten, dass jeder Behandlungstag eine hohe Strahlendosis in verschiedenen Teilen des Tumors appliziert. Gleichzeitig muss die unvermeidliche Strahlendosis in gesunden, den Tumor umgebenden Organen möglichst gleichmässig auf die Behandlungstage verteilt werden. Dadurch behält das gesunde Gewebe die Fähigkeit sich zwischen den Bestrahlungen zu regenerieren während sich der Behandlungseffekt im Tumor erhöht. Das Forschungsprojekt umfasst zwei zentrale Ziele: erstens die Weiterentwicklung der mathematischen Verfahren, um solche Behandlungen optimal zu planen; und zweitens das Verständnis der radiobiologischen Effekte zu verbessern.
Figure 1 illustrates the concept of spatiotemporal fractionation for treating a large cerebral arteriovenous malformation (AVM). The treatment consists of 4 fractions delivered with rotation therapy (VMAT or Tomotherapy). Each fraction delivers a high single fraction dose to a distinct part of the target volume.
Project title:
Spatiotemporal fractionation in radiotherapy
Principal investigator:
Jan Unkelbach, Department for Radiation Oncology, USZ
Contributing researchers:
Nathan Torelli, Martin Pruschy, Irene Vetrugno, Irma Telarovic
International collaborators:
David Papp, North Carolina State University, USA
Funding:
This project is supported by SNF: 2019 – 2023
Background:
In current clinical practice, most radiotherapy treatments are fractionated. This is motivated by the observation that most healthy tissues can tolerate a much higher total dose if the radiation is split into small fractions. On the other hand, fractionation typically requires that a higher total dose is delivered to the tumor to achieve the same level of response. Fractionation decisions therefore face the tradeoff between increasing the number of fractions to protect normal tissues and increasing the total dose to maintain the same level of tumor control.
The idea of Spatiotemporal fractionation:
In that regard, the ideal treatment would fractionate in normal tissues, and at the same time hypofractionate in the tumor. This appears to be impossible at first glance because the dose to normal tissues is an unavoidable consequence of delivering dose to the tumor. Generally, increasing the dose to the tumor in a given fraction will increase the dose to healthy tissues in that fraction. However, interestingly it is possible to achieve some degree of hypofractionation in parts of the tumor while exploiting the fractionation effect in normal tissues. The latter can be achieved by delivering distinct dose distributions in different fractions, a concept which is referred to a spatiotemporal fractionation.
Figure 1 illustrates the concept of spatiotemporal fractionation for treating a large cerebral arteriovenous malformation (AVM). The treatment consists of 4 fractions delivered with rotation therapy (VMAT or Tomotherapy). Each fraction delivers a high single fraction dose to a distinct part of the target volume. At the same time, each fraction creates a similar dose bath in the surrounding normal brain and thereby exploits the fractionation effect. Hence, partial hypofractionation in the target volume is achieved with more uniform fractionation in normal tissues, which yields a net improvement of the therapeutic ratio. This demonstrates that there may be a benefit of delivering different dose distributions in different fractions, purely motivated by fractionation effects rather than geometric changes of the patient.
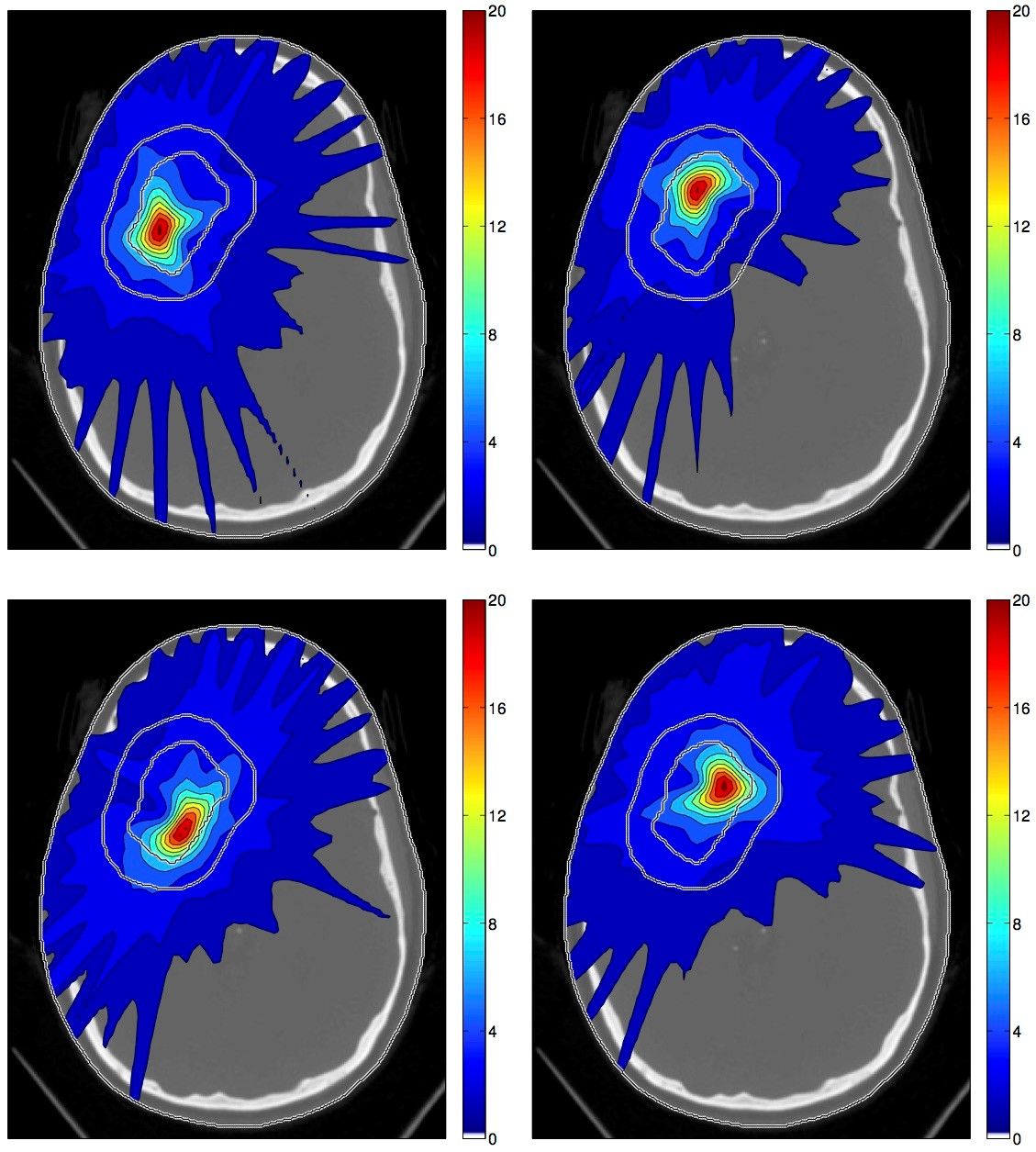
Figure 1
Aim 1: Development of treatment planning algorithms for spatiotemporal fractionation
Spatiotemporal fractionation leads to more complex treatment plans with distinct dose distributions for different fractions, which must be designed such that all fractions together deliver the prescribed dose to the tumor. To that end, novel mathematical optimization methods for treatment planning must be developed, which are based on cumulative biological dose rather than physical dose.
Aim 2: Estimating the potential benefit of spatiotemporal fractionation
We quantify the projected benefit of spatiotemporal fractionation over the current clinical practice of delivering the same dose distribution in every fraction. To that end, treatment planning studies are performed for treatment sites that are promising candidates for clinical applications of spatiotemporal fractionation. In particular, we investigate large liver tumors and brain lesions.
Aim 3: Pre-clinical investigation of the biology of spatiotemporal fractionation
Clinical experience is based on uniform fractionation where the entire tumor is treated with the same dose in every fraction. Spatiotemporal fractionation assumes that alternating parts of the tumor can be irradiated with high biologically effective doses in some fractions, but receive low doses in the remaining fractions. Using an image-guided high-precision small animal irradiation platform, we will test this assumption in experimental tumor models in mice, and thereby also explore novel radiobiological effects that may occur in the context of spatiotemporal fractionation and partial tumor irradiation.
Previous publications:
- J. Unkelbach, M. R. Bussiere, P. H. Chapman, J. S. Loeffler, and H. A. Shih, “Spatiotemporal Fractionation Schemes for Irradiating Large Cerebral Arteriovenous Malformations,” International journal of radiation oncology, biology, physics, vol. 95, no. 3, pp. 1067–74, Jul. 2016, doi: 10.1016/j.ijrobp.2016.02.001.
- J. Unkelbach, D. Papp, M. R. Gaddy, N. Andratschke, T. Hong, and M. Guckenberger, “Spatiotemporal fractionation schemes for liver stereotactic body radiotherapy,” Radiother Oncol, vol. 125, no. 2, pp. 357–364, Nov. 2017, doi: 10.1016/j.radonc.2017.09.003.
- M. R. Gaddy, S. Yildiz, J. Unkelbach, and D. Papp, “Optimization of spatiotemporally fractionated radiotherapy treatments with bounds on the achievable benefit,” Physics in medicine and biology, vol. 63, no. 1, p. 015036, Jan. 2018, doi: 10.1088/1361-6560/aa9975.
- J. Unkelbach and D. Papp, “The emergence of nonuniform spatiotemporal fractionation schemes within the standard BED model,” Medical physics, vol. 42, no. 5, pp. 2234–41, May 2015, doi: 10.1118/1.4916684.
- I. Telarovic et al., “Probing spatiotemporal fractionation on the preclinical level,” Phys Med Biol, vol. 65, no. 22, p. 22NT02, Nov. 2020, doi: 10.1088/1361-6560/abbb75.
Group leader: Stephanie Tanadini-Lang
Group members: Stefanie Ehrbar, Alexander Jöhl
Radiotherapy of liver tumors needs to account for motion of the liver. Real-time adaptive radiotherapy is an approach under investigation. In this approach, radiotherapy devices track the liver tumors continuously during treatment and compensate for the motion. To test and improve real-time adaptive radiotherapy, phantoms which represent the liver motions are required.
In a collaboration of the University Hospital Zürich, University of Zürich (USZ) and the Swiss Federal Institute of Technology (ETH), we have developed such an elastic liver phantom (ELPHA), which is dynamically deformable. This liver phantom features realistic liver motion, a time-resolved dosimetry system, and can be imaged using computer-tomography or ultrasound.
ELPHA consists of a deformable silicone torso including a silicone liver and vasculature, which can be imaged with ultrasound and radiography. Respiration is mimicked by compression of the torso. Electromagnetic transponders inside the phantom allow to measure its displacement in real-time. The phantom has been shown to reproduce liver motion traces with submillimeter accuracy. Plastic scintillation dosimeters (PSD) are inserted to the liver for a time-resolved measurement of radiation dose applied to ELPHA. With this dosimetry system and the ability of respiratory deformation, real-time adaptive treatments can be tested, verified and improved.

Collaborations:
- ETH Zurich, Product Development Group Zurich, Department of Mechanical and Process Engineering
- ETH Zurich, Computer-assisted Applications in Medicine, Department of Information Technology and Electrical Engineering