Endocrine and Age-related Myopathies
AG Krützfeldt / Hormon- und altersabhängige Muskelerkrankungen
Wir untersuchen molekulare Mechanismen der hormonabhängigen Regeneration und Funktion des Skelettmuskels und ihre Veränderungen während des Alterns.
Der Skelettmuskel ist das grösste Organ des Menschen und für die Gesundheit von zentraler Bedeutung. Dies zeigt sich an den positiven Wirkungen von körperlicher Aktivität, aber auch an vielen Erkrankungen, bei denen die Regeneration oder Funktion des Muskels eingeschränkt sind. Hierzu zählen zum Beispiel der Diabetes mellitus Typ 2, bei dem der Skelettmuskel nicht mehr ausreichend auf Insulin anspricht, die altersabhängige Abnahme der Muskelmasse (Sarkopenie) sowie Veränderungen der Muskulatur nach Ersatztherapien mit Hormonen wie Wachstums- oder Schilddrüsenhormon oder bei einem Überschuss an Kortison. Unsere Arbeitsgruppe untersucht, welche Rolle mikroRNAs bei diesen Veränderungen spielen. mikroRNAs sind kleine RNA Moleküle, die erst vor wenigen Jahren entdeckt wurden. Sie sind an der Regulation der Proteinbildung in der Zelle beteiligt, indem sie sich an andere RNA Moleküle anbinden und ihre Umschreibung in Protein reduzieren. Unser Ziel ist es durch ein besseres Verständnis der molekularen Grundlagen von hormonabhängigen Muskelerkrankungen eine frühzeitige Erkennung zu ermöglichen sowie neue therapeutische Ansätze aufzuzeigen.
Research Topics
Skeletal muscle regeneration by adult muscle stem cells
Adult muscle stem cells, also termed satellite cells or myogenic progenitors (MPs), are critical for the maintenance and regeneration of functional myofibers throughout life. MPs are responsible for a remarkable plasticity of skeletal muscle with 1-2% of myonuclei being replaced every week. In fact, during postnatal growth, the majority of adult myonuclei are derived from adult muscle stem cells. Adult muscle stem cells are therefore a key target for therapeutic interventions in muscle-related diseases such as type 2 diabetes, sarcopenia or endocrine myopathies. We are using mouse models for muscle regeneration and work with primary muscle cells from mice and humans to better understand the role of microRNAs in this process. We have recently developed the first inducible muscle stem cell-specific microRNA knockout mouse which demonstrated the critical role of miR-29a for muscle regeneration (Galimov A et al, 2016). We have also developed protocols to inhibit microRNAs during muscle regeneration using intramuscular injections of antagomirs (Mizbani A et al, 2016).
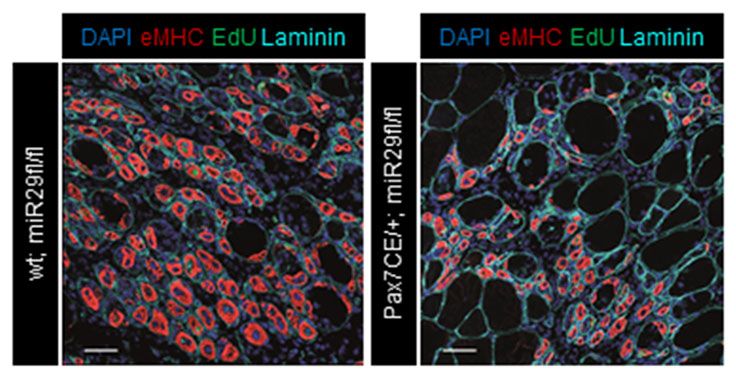
Effect of an inducible knockout of miR-29a in adult muscle stem cells on formation of newly formed myofibers (red) during muscle regeneration (from Galimov A et al, 2016).
Skeletal muscle metabolism and mitochondrial dysfunction
Skeletal muscle is fundamental to whole-body metabolism as 30-70% of postprandial glucose is utilized by this tissue. Skeletal muscle is also essential for lipid metabolism as it covers 80-90% of its energy requirements in the resting state through the uptake and oxidation of fatty acids. Our group is interested to identify microRNAs that can be targeted for the generation of metabolically active myofibers and thus improvement of whole body glucose homeostasis. To achieve this aim, we are employing genetic screens that are based on fluorescence assays and confocal microscopy in human myotubes.
Hormonal control of myokine secretion in skeletal muscle
Insulin resistance and type 2 diabetes (T2D) are at highest prevalence in people aged 65 years or older and population aging is now considered a major driver to the epidemic rise of T2D. Skeletal muscle is one of the organs that is most severely affected by the aging process as muscle mass and function steadily decline starting after 40 years of age. A better understanding of this process at the molecular level is needed to design therapeutic strategies to prevent the adverse effects of aging. We are studying the regulation of secreted peptides, termed myokines, in skeletal muscle by hormone-dependent microRNA expression. We are employing different techniques of molecular biology to inhibit microRNAs in muscle cells combined with analysis of secreted peptides using mass spectrometry.
Ectopic fat formation in adult skeletal muscle
Recent evidence points to unexpected roles of adipose tissue at ectopic locations such as adult skeletal muscle, where white adipose tissue is a critical regulator of muscle regeneration, while brown adipose tissue between muscle bundles protects against obesity. We have recently shown that intramuscular adipogenesis is associated with increased expression of the brown adipocyte marker Uncoupling protein-1 (UCP1) in skeletal muscle from obesity-resistant mice (Gorski et al, 2017). We also demonstrated that fibro/adipogenic progenitors (FAPs) provide a likely source for intramuscular adipocytes expressing UCP1 under control of both genetic and hormonal factors. Therefore, FAPs constitute a possible target for therapies aiming at the browning of intramuscular adipose tissue and the metabolic improvement of skeletal muscle affected by fatty infiltration. In this project we aim to understand how different types of adipocytes in adult skeletal muscle regulate energy expenditure and impact on muscle regeneration.
Glycerol-injected tibialis anterior muscle
UCP1 COX IV
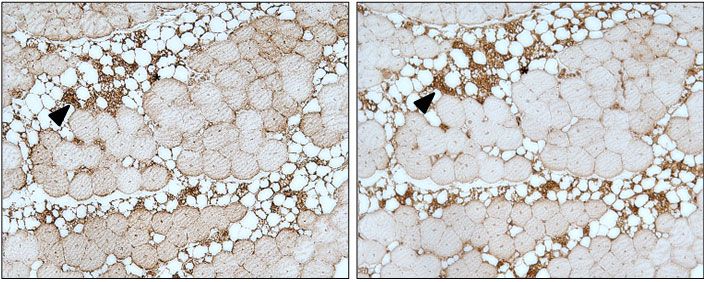
Appearance of UCP1+ and COXIV+ adipocytes in intramuscular fat tissue of Sv129 mice after beta-3-adrenergic stimulation (from Gorski et al, 2017)
Cooperations
- Molecular mechanisms of sarcopenia: Prof Dr Heike A. Bischoff-Ferrari, Centre on Aging and Mobility, University Hospital Zurich
- Role of intramuscular adipocytes: Prof Dr Christian Wolfrum, Department of Health Sciences and Technologies, ETH Zurich
- Impact of lipoprotein particles on mitochondrial function: Prof Dr Arnold von Eckardstein, Institute of Clinical Chemistry, University Hospital Zurich
Fundings
- SNF grant 31003A_182716: The role of microRNAs in age-related and endocrine myopathies (12/18-11/23)
- Philhuman Stiftung
- Uniscientia Foundation
microRNA-501 controls myogenin+/CD74+ myogenic progenitor cells during muscle regeneration.
Fahrner A, Luca E, Krützfeldt J.
Molecular Metabolism. 2023 May;71:101704. doi: 10.1016/j.molmet.2023.101704.
Activation of PDGF signaling in the adult muscle stem cell niche in patients with type 2 diabetes mellitus.
Fahrner A, Laiferova NA, Ukropcová B, Ukropec J, Krützfeldt J.
J Clin Endocrinol Metab. 2023 Jan 26:dgad041. doi: 10.1210/clinem/dgad041
Growth hormone/IGF-I-dependent signaling restores decreased expression of the myokine SPARC in aged skeletal muscle
Mathes S, Fahrner A, Luca E, and Krützfeldt J. J Mol Med (Berl). 2022, 10.1007/s00109-022-02260-w
FGF-2-dependent signaling activated in aged human skeletal muscle promotes intramuscular adipogenesis
Mathes S, Fahrner A, Ghoshdastider U, Rüdiger HA, Leunig M, Wolfrum C, and Krützfeldt J. PNAS. 2021 Sep 14;118(37):e2021013118. doi: 10.1073.
Genetic deletion of microRNA biogenesis in muscle cells reveals a hierarchical non-clustered network that controls focal adhesion signaling during muscle regeneration
Luca E, Turcekova K, Hartung A, Mathes S, Rehrauer H, and Krützfeldt J.
Molecular Metabolism. 2020 Jun; 36: 100967. doi: 10.1016
UCP1 expression in adipocytes derived from skeletal muscle fibro/adipogenic progenitors is under genetic and hormonal control
Gorski T, Mathes S, and Krützfeldt J.
J Cachexia Sarcopenia Muscle. 2018 Apr;9(2):384-399.
microRNA deep sequencing in two adult stem cell populations identifies miR-501 as novel regulator of myosin heavy chain during muscle regeneration
Amir Mizbani, Edlira Luca, Elisabeth J. Rushing, and Jan Krützfeldt
Development. 2016 Nov 15;143(22):4137-4148.
Strategies to use microRNAs as therapeutic targets
Jan Krützfeldt
Best Pract Res Clin Endocrinol Metab. 2016 Oct;30(5):551-561. doi: 10.1016/j.beem.2016.07.004.
microRNA-29a in adult muscle stem cells controls skeletal muscle regeneration during injury and exercise downstream of fibroblast growth factor-2
Galimov A, Merry TL, Luca E, Rushing EJ, Mizbani A, Turcekova K, Hartung A, Croce CM, Ristow M, and Krützfeldt J
Stem Cells. 2016 Jan 5. doi: 10.1002/stem.2281. [Epub ahead of print]
Growth hormone replacement therapy regulates microRNA-29a and targets involved in insulin resistance
Galimov A1, Hartung A, Trepp R, Mader A, Flück M, Linke A, Blüher M, Christ E, Krützfeldt J.
J Mol Med (Berl). 2015 Dec;93(12):1369-79. doi: 10.1007/s00109-015-1322-y.
MicroRNA-194 is a target of transcription factor 1 (Tcf1, Hnf1 α in adult liver and controls expression of frizzled-6
Krützfeldt J, Rösch N, Hausser J, Manoharan M, Zavolan M, Stoffel M.
Hepatology. 2012 Jan;55(1):98-107
Specificity, duplex degradation and subcellular localization of antagomirs
Krützfeldt J, Kuwajima S, Braich R, Rajeev KG, Pena J, Tuschl T, Manoharan M, Stoffel M.
Nucleic Acids Res. 2007 April; 35(9):2885-92
MicroRNAs: A new class of regulatory genes affecting metabolism
Krützfeldt J, Stoffel M.
Cell Metab. 2006 Jul;4(1):9-12
Silencing of microRNAs in vivo with ‚antagomirs‘
Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M.
Nature. 2005 Dec 1;438(7068):685-9
Strategies to determine the biological function of microRNAs
Krützfeldt J, Poy MN, Stoffel M.
Nat Genet. 2006 Jun;38 Suppl 1:S14-9


